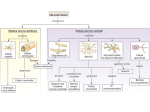
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Photoreception
Axon guidance wikipedia , lookup
Subventricular zone wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Development of the nervous system wikipedia , lookup
Optogenetics wikipedia , lookup
NMDA receptor wikipedia , lookup
Synaptogenesis wikipedia , lookup
Signal transduction wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Photoreception: The vertebrate retina is a thin sheet of tissue lining the posterior part of the eye. It is highly organized and consists of several layers of neurons (Fig. 1). The outermost layer comprises photosensitive cells, called photoreceptors. The photoreceptors are signal transducers; they transduce the absorption of light into an electrical signal. Photoreceptors come in two flavours: rods and cones. Rods are exquisitely sensitive to light and can even detect single photons. They allow vision in dim light. In contrast, cones are less (30-100-fold) sensitive to light than rods. They are used for vision during day light and for colour vision. The highest density of cones is found in the center of the retina, the area centralis. In humans, in other primates and in some birds, the very center of the retina is designed as a pit and is called fovea. Both cell types, rods and cones, consist of four major parts - an outer segment, an inner segment that are connected by a thin rudimentary cilium, a soma, and an axon with a synaptic region (Fig. 2). The outer segment houses the molecular components for light absorption and generation of electrical signals. The inner segment harbours the machinery for protein synthesis and energy production. Figure 2: Photoreceptor cells. Schematic drawing of rod and cone photoreceptor cells. The primary events of photoreception take place in the outer segment of the cell, shown in brown. The electrical light response is transmitted to other neurons through the synaptic region. The outer segments of rods and cones are densely packed with stacks of membrane, the discs. The disc membrane harbours the visual pigment, rhodopsin. The absorption of light triggers a photochemical reaction which isomerizes the chromophore of rhodopsin, 11-cis retinal, and thereby initiates a sequence of conformational changes in the protein part of the molecule (Fig. 3). One intermediate of the photochemical cycle of rhodopsin, metarhodopsin II, is formed within a few milliseconds and persists for several minutes. This activated form of rhodopsin interacts with a trimeric GTP-binding protein, transducin and thereby promotes the exchange of bound GDP for GTP at the α-subunit of transducin. As a consequence, the α-subunit dissociates from the βγ-subunits of transducin. Subsequently, the α-subunit of transducin binds to and thereby removes an inhibitory subunit from a phosphodiesterase (PDE). The PDE hydrolyzes the intracellular messenger of photoreception: cGMP to GMP. Figure 3: Photochemistry of rhodopsin. A) Illumination triggers the isomerization of 11-cis-retinal to all-transretinal. B) Upon illumination, rhodopsin undergoes a sequence of conformational changes that produce meta-rhodopsin II, a stable intermediate of the photocycle that activates the next protein in the transduction cascade, transducin. What is the function of cGMP in rod and cone photoreceptors (Fig. 4)? In the dark, the intracellular concentration of cGMP is high. The second messenger binds to ion channels in the plasma membrane of the photoreceptor cell. These channels are directly gated open by cGMP and are therefore, referred to as cyclic nucleotide-gated (CNG) ion channels. A few percent of CNG channels are kept open in the dark by binding of cGMP and allow the influx of ions into the outer segment. This influx is primarily carried by sodium (Na +) ions, but a small fraction is also carried by Ca 2+ ions. At the same time K+ ions leave the inner segment through potassium (K+) channels. Due to this circulating current along the rod photoreceptor, the so-called dark-current, the membrane potential of the photoeceptor cell is slightly depolarized. This depolarization suffices to release the neurotransmitter glutamate from the synaptic terminal onto postsynaptic cells in the retina, the horizontal and bipolar cells. Upon illumination, the cGMP is hydrolyzed by the PDE. The cGMP concentration decreases and CNG channels close. Since current stops flowing into the cell, the cell hyperpolarizes and glutamate release ceases. The change in neurotransmitter release is registered by bipolar cells, which relay this information onto ganglion cells. The ganglion cells then convey the information to the brain. Figure 4: Electrical response of photoreceptors to illumination. In darkness, CNG channels in the plasma membrane of photoreceptor outer segments are kept open by cGMP and the cell depolarizes (left). Upon illumination, CNG channels close and the photoreceptor hyperpolarizes (right). Recovery of the photoreceptor from the light response and adjustment of light sensitivity is achieved by several feedback mechanisms. Ca2+ ions play a major role in recovery and light adaptation. Upon illumination, Ca2+ entry through the CNG channel ceases, whereas a Na+/Ca2+-K+ exchanger continues to export Ca2+ ions from the cell. The ensuing decrease of the intracellular Ca2+ concentration triggers recovery and adaptation (Fig. 5). Ca2+ controls the synthesis of cGMP by guanylate cyclase (GC). The enzymatic activity is controlled by specific Ca 2+-binding proteins (GCAP's). Furthermore, inactivation of enzymatically active rhodopsin is achieved by phosphorylation through rhodopsin kinase. This process involves another Ca2+-binding protein: recoverin. Finally, the ligand sensitivity of the CNG channel is controlled by a third Ca2+-binding protein: calmodulin. Figure 5: Acivation and recovery of the phototransduction cascade. Two cycles necessary for transduction and for adaptation control and fine-tune each other. Through CNG channels, cGMP controls the influx of Ca2+, at the same time via GCAP and GC, Ca2+ controls the cGMP concentration. From: http://www.fz-juelich.de/ibi/ibi-1/Photoreception Glutamate and glutamate receptors in the vertebrate retina 1. General overview of synaptic transmission. Cells communicate with each other electrically, through gap junctions, and chemically, using neurotransmitters. Chemical synaptic transmission allows nerve signals to be exchanged between cells which are electrically isolated from each other. The chemical messenger, or neurotransmitter, provides a way to send the signal across the extracellular space, from the presynaptic neuron to the postsynaptic cell. The space is called a cleft and is typically more than 10 nanometers across. Neurotransmitters are synthesized in the presynaptic cell and stored in vesicles in presynaptic processes, such as the axon terminal. When the presynaptic neuron is stimulated, calcium channels open and the influx of calcium ions into the axon terminal triggers a cascade of events leading to the release of neurotransmitter. Once released, the neurotransmitter diffuses across the cleft and binds to receptors on the postsynaptic cell, allowing the signal to propagate. Neurotransmitter molecules can also bind onto presynaptic autoreceptors and transporters, regulating subsequent release and clearing excess neurotransmitter from the cleft. Compounds classified as neurotransmitters have several characteristics in common (reviewed in Massey, 1990, Erulkar, 1994). Briefly, (1) the neurotransmitter is synthesized, stored, and released from the presynaptic terminal. (2) Specific neurotransmitter receptors are localized on the postsynaptic cells, and (3) there exists a mechanism to stop neurotransmitter release and clear molecules from the cleft. Common neurotransmitters in the retina are glutamate, GABA, glycine, dopamine, and acetylcholine. Neurotransmitter compounds can be small molecules, such as glutamate and glycine, or large peptides, such as vasoactive intestinal peptide (VIP). Some neuroactive compounds are amino acids, which also have metabolic functions in the presynaptic cell. Fig. 1. Structure of the glutamate molecule (39 K jpeg image) Glutamate (Fig. 1) is believed to be the major excitatory neurotransmitter in the retina. In general, glutamate is synthesized from ammonium and alpha-ketoglutarate (a component of the Krebs Cycle) and is used in the synthesis of proteins, other amino acids, and even other neurotransmitters (such as GABA; Stryer, 1988). Though glutamate is present in all neurons, only a few are glutamatergic, releasing glutamate as their neurotransmitter. Neuroactive glutamate is stored in synaptic vesicles in presynaptic axon terminals (Fykse and Fonnum, 1996). Glutamate is incorporated into the vesicles by a glutamate transporter located in the vesicular membrane. This transporter selectively accumulates glutamate through a sodium-independent, ATP-dependent process (Naito and Ueda, 1983, Tabb and Ueda, 1991, Fykse and Fonnum, 1996), resulting in a high concentration of glutamate in each vesicle. Neuroactive glutamate is classified as an excitatory amino acid (EAA) because glutamate binding onto postsynaptic receptors typically stimulates, or depolarizes, the postsynaptic cells. 2. Histological techniques identify glutamatergic neurons. Fig. 2. Glutamate immunoreactivity (39 K jpeg image) Using immunocytochemical techniques, neurons containing glutamate are identified and labeled with a glutamate antibody. In the retina, photoreceptors, bipolar cells, and ganglion cells are glutamate immunoreactive (Ehinger et al, 1988, Marc et al., 1990, Van Haesendonck and Missotten, 1990, Kalloniatis and Fletcher, 1993, Yang and Yazulla, 1994, Jojich and Pourcho, 1996) (Fig. 2). Some horizontal and/or amacrine cells can also display weak labeling with glutamate antibodies (Ehinger et al., 1988, Marc et al., 1990, Jojich and Pourcho, 1996; Yang, 1996). These neurons are believed to release GABA, not glutamate, as their neurotransmitter (Yazulla, 1986), suggesting the weak glutamate labeling reflects the pool of metabolic glutamate used in the synthesis of GABA. This has been supported by the results from double-labeling studies using antibodies to both GABA and glutamate: glutamatepositive amacrine cells also label with the GABA antibodies (Jojich and Pourcho, 1996, Yang, 1996). Fig. 3. Autoradiogram of glutamate uptake through glutamate transporters (39 K jpeg image) Photoreceptors, which contain glutamate, actively take up radiolabeled glutamate from the extracellular space, as do Muller cells (Fig. 3) (Marc and Lam, 1981; Yang and Wu, 1997). Glutamate is incorporated into these cell types through a high affinity glutamate transporter located in the plasma membrane. Glutamate transporters maintain the concentration of glutamate within the synaptic cleft at low levels, preventing glutamate-induced cell death (Kanai et al., 1994). Though Muller cells take up glutamate, they do not label with glutamate antibodies (Jojich and Pourcho, 1996). Glutamate incorporated into Muller cells is rapidly broken down into glutamine, which is then exported from glial cells and incorporated into surrounding neurons (Pow and Crook, 1996). Neurons can then synthesize glutamate from glutamine (Hertz, 1979, Pow and Crook, 1996). Thus, histological techniques are used to identify potential glutamatergic neurons by labeling neurons containing glutamate (through immunocytochemistry) and neurons that take up glutamate (through autoradiography). To determine if these cell types actually release glutamate as their neurotransmitter, however, the receptors on postsynaptic cells have to be examined. 3. Glutamate receptors. Once released from the presynaptic terminal, glutamate diffuses across the cleft and binds onto receptors located on the dendrites of the postsynaptic cell(s). Multiple glutamate receptor types have been identified. Though glutamate will bind onto all glutamate receptors, each receptor is characterized by its sensitivity to specific glutamate analogues and by the features of the glutamate-elicited current. Glutamate receptor agonists and antagonists are structurally similar to glutamate (Fig. 4), which allows them to bind onto glutamate receptors. These compounds are highly specific and, even in intact tissue, can be used in very low concentrations because they are poor substrates for glutamate uptake systems (Tachibana and Kaneko, 1988, Schwartz and Tachibana, 1990). Fig. 4. Glutamate receptor agonists and antagonists (39 K jpeg image) Two classes of glutamate receptors (Fig. 5) have been identified: (1) ionotropic glutamate receptors, which directly gate ion channels, and (2) metabotropic glutamate receptors, which may be coupled to an ion channel or other cellular functions via an intracellular second messenger cascade. These receptor types are similar in that they both bind glutamate and glutamate binding can influence the permeability of ion channels. However, there are several differences between the two classes. Fig. 5. Ionotropic and metabotropic glutamate receptors and channels (39 K jpeg image) 4. Ionotropic glutamate receptors. Glutamate binding onto an ionotropic receptor directly influences ion channel activity because the receptor and the ion channel form one complex (Fig. 5a). These receptors mediate fast synaptic transmission between neurons. Each ionotropic glutamate receptor, or iGluR, is formed from the co-assembly of individual subunits. The assembled subunits may or may not be homologous, with the different combinations of subunits resulting in channels with different characteristics (Keinanen et al., 1990, Verdoorn et al., 1991, Moyner et al., 1992; Nakanishi, 1992, Ozawa and Rossier, 1996). Fig. 6. Comparison between NMDA and non-NMDA receptors(39 K jpeg image) Two iGluR types (see Fig. 6) have been identified: (1) NMDA receptors, which bind glutamate and the glutamate analogue N-Methyl-D-Aspartate (NMDA) and (2) non-NMDA receptors, which are selectively agonized by kainate, AMPA, and quisqualate, but not NMDA. Non-NMDA receptors. Glutamate binding onto a non-NMDA receptor opens non-selective cation channels more permeable to sodium (Na+) and potassium (K+) ions than calcium (Ca+2) (Mayer and Westbrook, 1987). Glutamate binding elicits a rapidly activating inward current at membrane potentials negative to 0 mV, and an outward current at potentials positive to 0 mV. Kainate, quisqualate, and AMPA (alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) are the specific agonists at these receptors; CNQX (6-cyano-7-nitroquinoxaline-2,3-dione), NBQX (1,2,3,4-tetrahydro-6nitro-2,3-dione-benzo[f]quinoxaline-7-sulfonamide), and DNQX (6,7-dinitroquinoxaline-2,3-dione) are the antagonists. Fig. 7. Whole-cell patch clamp to show quisqualate and kainate gated currents(39 K jpeg image) In retina, non-NMDA receptors have been identified on horizontal cells, OFF-bipolar cells, amacrine cells, and ganglion cells (see below). Patch clamp recordings (Gilbertson et al., 1991, Zhou et al., 1993, Boos et al., 1993, Cohen and Miller, 1994, Yu and Miller, 1995) indicate that AMPA, quisqualate, and/or kainate application can evoke currents in these cells. However, the kinetics of the ligand-gated currents differ. AMPA - and quisqualate-elicited currents rapidly desensitize; whereas, kainate-gated currents do not (Fig. 7a). The desensitization at AMPA/quisqualate receptors can be reduced (Fig. 7b) by adding cyclothiazide (Yamada and Tang, 1993), which stabilizes the receptor in an active (or non-desensitized) state (Yamada and Tang, 1993, Kessler et al., 1996). Each non-NMDA receptor is formed from the co-assembly of several subunits (Boulter et al., 1990, Nakanishi et al., 1990, Nakanishi, 1992). To date, seven subunits (named GluR1 through GluR7) have been cloned (Hollmann et al., 1989, Boulter et al., 1990, Keinanen et al., 1990, Nakanishi et al., 1990, Bettler et al., 1990, 1992, Egebjerg et al., 1991). Expression of subunit clones in Xenopus oocytes revealed that GluR5, GluR6, and GluR7 (along with subunits KA1 and KA2) coassemble to form kainate(-preferring) receptors; whereas, GluR1, GluR2, GluR3, and GluR4 are assembled into AMPA(preferring) receptors (Nakanishi, 1992). NMDA receptors. Glutamate binding onto an NMDA receptor also opens non-selective cation channels, resulting in a conductance increase. However, the high conductance channel associated with these receptors is more permeable to Ca+2 than Na+ ions (Mayer and Westbrook, 1987) and NMDA-gated currents typically have slower kinetics than kainate- and AMPA-gated channels. As the name suggests, NMDA is the selective agonist at these receptors. The compounds MK-801, AP-5 (2-amino-5-phosphonopentanoic acid), and AP-7 (2-amino-7-phosphoheptanoic acid) are NMDA receptor antagonists. NMDA receptors are structurally complex, with separate binding sites for glutamate, glycine, magnesium ions (Mg+2), zinc ions (Zn+2), and a polyamine recognition site (Fig. 6b). There is also an antagonist binding site for PCP and MK-801 (Lodge, 1997). The glutamate, glycine, and magnesium binding sites are important for receptor activation and gating of the ion channel. In contrast, the zinc and polyamine sites are not needed for receptor activation, but affect the efficacy of the channel. Zinc blocks the channel in a voltage-independent manner (Westbrook and Mayer, 1987). The polyamine site (Ransom and Stec, 1988, Williams et al., 1994) binds compounds such as spermine or spermidine, either potentiating (Ranson and Stec, 1988; Williams et al., 1994) or inhibiting (Williams et al., 1994) the activity of the receptor, depending on the combination of subunits forming each NMDA receptor (Williams et al., 1994). To date, five subunits (NR1, NR2a, N2b, N2c, and N2d) of NMDA receptors have been cloned (Moriyoshi et al., 1991, Ikeda et al., 1992, Katsuwada et al., 1992, Meguro et al., 1992, Ishii et al., 1993). As with non-NMDA receptors, NMDA receptor subunits can co-assemble as homomers (i.e., five NR1 subunits; Moyner et al., 1992, Moriyoshi et al., 1992) or heteromers (one NR1 + four NR2 subunits; Meguro et al., 1992, Katsuwada et al., 1992, Moyner et al., 1992, Ishii et al., 1993). However, all functional NMDA receptors express the NR1 subunit (Moyner et al., 1992, Nakanishi, 1992, Ishii et al., 1993). Fig. 8. NMDA receptor activation(39 K jpeg image) The glutamate, glycine, and Mg+2 binding sites confer both ligand-gated and voltage-gated properties onto NMDA receptors. NMDA receptors are ligand-gated because the binding of glutamate (ligand) is required to activate the channel. In addition, micromolar concentrations of glycine must also be present (Fig. 8) (Johnson and Ascher, 1987, Kleckner and Dingledine, 1988). The requirement for both glutamate and glycine makes them co-agonists (Kleckner and Dingledine, 1988) at NMDA receptors. Mg+2 ions provide a voltage-dependent block of NMDA-gated channels (Nowak et al., 1984). This can be seen in the current-voltage (I-V) relationship presented in Fig. 9 (from Nowak et al., 1984). Fig. 9. Mg+2 ions block NMDA receptor channels(39 K jpeg image) I-V curves plotted from currents recorded in the presence of Mg+2 have a characteristic J-shape (dotted line); whereas, a linear relationship is calculated in Mg+2-free solutions (solid line). At negative membrane potentials, Mg+2 ions occupy the binding site causing less current to flow through the channel. As the membrane depolarizes, the Mg+2 block is removed (Nowak et al., 1984). Retinal ganglion cells and some amacrine cell types express functional NMDA receptors in addition to non-NMDA receptors (i.e., Massey and Miller, 1988, 1990, Mittman et al., 1990, Dixon and Copenhagen 1992, Diamond and Copenhagen, 1993, Cohen and Miller, 1994). The currents elicited through these different iGluR types can be distinguished pharmacologically. Non-NMDA receptor antagonists block a transient component of the ganglion cell light response; whereas, NMDA receptor antagonists block a more sustained component (Mittman et al., 1990, Diamond and Copenhagen, 1993, Hensley et al., 1993, Cohen and Miller, 1994). These findings suggest the currents elicited through co-localized NMDA and non-NMDA receptors mediate differential contributions to the ON- and OFF-light responses observed in ganglion cells (i.e., Diamond and Copenhagen, 1993). 5. Metabotropic glutamate receptors. Unlike ionotropic receptors, which are directly linked to an ion channel, metabotropic receptors are coupled to their associated ion channel through a second messenger pathway. Ligand (glutamate) binding activates a G-protein and initiates an intracellular cascade (Nestler and Duman, 1994). Metabotropic glutamate receptors (mGluRs) are not coassembled from multiple subunits, but are one polypeptide (Fig. 5b). To date, eight mGluRs (mGluR1-mGluR8) have been cloned (Houamed et al., 1991, Masu et al., 1991, Abe et al., 1992, Tanabe et al., 1992, Nakajima et al., 1993, Saugstad et al., 1994, Duvoisin et al., 1995). These receptors are classified into three groups (I, II, and III) based on structural homology, agonist selectivity, and their associated second messenger cascade (Table 1, end of chapter) (reviewed in Nakanishi, 1994, Knopfel et al., 1995, Pin and Duvoisin, 1995, Pin and Bockaert, 1995). In brief, Group I mGluRs (mGluR1 and mGluR5) are coupled to the hydrolysis of fatty acids and the release of calcium from internal stores. Quisqualate and trans-ACPD are Group I agonists. Group II (mGluR2 and mGluR3) and Group III (mGluRs 4, 6, 7, and 8) receptors are considered inhibitory because they are coupled to the downregulation of cyclic nucleotide synthesis (Pin and Duvoisin, 1995). L-CCG-1 and trans-ACPD agonize Group II receptors; L-AP4 (also called APB) selectively agonizes Group III receptors. In situ hybridization studies have revealed that the mRNAs encoding Group I, II, and III mGluRs are present in retina (see below); however, with the exception of the APB receptor, the function of all these receptor types in retina has not been characterized. APB receptor. In contrast to non-NMDA and NMDA receptors, glutamate binding onto an APB receptor elicits a conductance decrease (Slaughter and Miller, 1981, Nawy and Copenhagen, 1987, 1990) due to the closure of cGMP-gated non-selective cation channels (Nawy and Jahr, 1990) (Fig. 10). Fig. 10.Whole-cell current traces to show kinetics of APB receptor gated currents(39 K jpeg image) APB application selectively blocks the ON-pathway in the retina (Fig. 11) (Slaughter and Miller, 1981), i.e., ON-bipolar cell responses and the ON-responses in amacrine cells (Taylor and Wassle, 1995) and ganglion cells (Cohen and Miller, 1994, Kittila and Massey, 1995, Jin and Brunken, 1996) are eliminated by APB. Experimental evidence (Slaughter and Miller 1981, Massey et al., 1983) suggests the APB receptor is localized to ON-bipolar cell dendrites. Inhibition of amacrine and ganglion cell light responses, therefore, is due to a decrease in the input from ON-bipolar cells, not a direct effect on postsynaptic receptors. Fig. 11. Intracellular recordings to show that APB selectively antagonizes the ON-pathways (39 K jpeg image) APB (2-amino-4-phosphobutyric acid, also called L-AP4) is the selective agonist for all Group III mGluRs (mGluR4, 6, 7, and 8). So, which is the APB receptor located on ON-bipolar cell dendrites? MGluR4, 7, and 8 expression has been observed in both the inner nuclear layer and the ganglion cell layer (Duvoisin et al., 1995, Hartveit et al., 1995) suggesting these mGluRs are associated with more than one cell type. In contrast, mGluR6 expression has been localized to the INL (Nakajima et al., 1993, Hartveit et al., 1995) and the OPL (Nomura et al., 1994) where bipolar cell somata and dendrites are located. Furthermore, ON-responses are abolished in mice lacking mGluR6 expression (Masu et al., 1995). These mutants also display abnormal ERG b-waves, suggesting an inhibition of the ONretinal pathway at the level of bipolar cells (Masu et al., 1995). Taken together, these findings suggest the APB receptor on ON-bipolar cells is mGluR6. 6. Glutamate transporters and transporter-like receptors. Glutamate transporters have been identified on photoreceptors (Marc and Lam, 1981, Tachibana and Kaneko, 1988, Eliasof and Werblin, 1993) and Muller cells (Marc and Lam, 1981, Yang and Wu, 1997). From glutamate labeling studies, the average concentration of glutamate in photoreceptors, bipolar cells, and ganglion cells is 5mM (Marc et al. 1990). Physiological studies using isolated cells indicate that only µM levels of glutamate are required to activate glutamate receptors (i.e., Aizenman et al., 1988, Zhou et al., 1993, Sasaki and Kaneko, 1996). Thus, the amount of glutamate released into the synaptic cleft is several orders of magnitude higher than the concentration required to activate most postsynaptic receptors. High affinity glutamate transporters located on adjacent neurons and surrounding glial cells rapidly remove glutamate from the synaptic cleft to prevent cell death (Kanai et al., 1994). Five glutamate transporters, EAAT-1 (or GLAST), EAAT-2 (or GLT-1), EAAT-3 (or EAAC-1), EAAT-4, and EAAT-5, have been cloned (Kanai and Hediger, 1992, Pines et al., 1992, Fairman et al., 1995, Schultz and Stell, 1996, Arriza et al., 1997, Kanai et al., 1997). Glutamate transporters are pharmacologically distinct from both iGluRs and mGluRs. L-glutamate, L-aspartate, and Daspartate are substrates for the transporters (Brew and Attwell, 1987, Tachibana and Kaneko, 1988, Eliasof and Werblin, 1993); glutamate receptor agonists (Brew and Attwell, 1987, Tachibana and Kaneko, 1988, Schwartz and Tachibana, 1990, Eliasof and Werblin, 1993) and antagonists (Barbour et al., 1991, Eliasof and Werblin, 1993) are not. Glutamate uptake can be blocked by the transporter blockers dihydrokainate (DHKA) and DL-threo-beta-hydroxyaspartate (HA) (Barbour et al., 1991, Eliasof and Werblin 1993). Fig. 12 Glutamate transporters in Muller cells are electrogenic (39 K jpeg image) Glutamate transporters incorporate glutamate into Muller cells along with the co-transport of three Na+ ions (Brew and Attwell, 1987, Barbour et al., 1988) and the antiport of one K+ ion (Barbour et al., 1988, Bouvier et al., 1992) and either one OH- or one HCO3- ion (Bouvier et al., 1992) (Fig. 12). The excess sodium ions generate a net positive inward current which drives the transporter (Brew and Attwell, 1987, Barbour et al., 1988). More recent findings indicate a glutamateelicited chloride current is also associated with some transporters (Eliasof and Jahr, 1996, Arriza et al., 1997). It should be noted that the glutamate transporters located in the plasma membrane of neuronal and glial cells (discussed in this section) are different from the glutamate transporters located on synaptic vesicles within presynaptic terminals (see section 1). The transporters in the plasma membrane transport glutamate in a Na+- and voltage-dependent manner independent of chloride (Brew and Attwell, 1987, Barbour et al., 1988, Kanai et al., 1994). L-glutamate, L-aspartate, and D-aspartate are substrates for these transporters (i.e., Brew and Attwell, 1987). In contrast, the vesicular transporter selectively concentrates glutamate into synaptic vesicles in a Na+-independent, ATP-dependent manner (Naito and Ueda, 1983, Tabb and Ueda, 1991, Fykse and Fonnum, 1996) that requires chloride (Tabb and Ueda, 1991, Fykse and Fonnum, 1996). Glutamate receptors with transporter-like pharmacology have been described in photoreceptors (Picaud et al., 1995a, b, Grant and Werblin, 1996) and ON-bipolar cells (Grant and Dowling 1995, 1996). These receptors are coupled to a chloride current. The pharmacology of these receptors is similar to that described for glutamate transporters, as the glutamateelicited current is (1) dependent upon external Na+, (2) reduced by transporter blockers, and (3) insensitive to glutamate agonists and antagonists. However, altering internal Na+ concentration does not change the reversal potential (Picaud et al., 1995b) or the amplitude (Grant and Werblin, 1995, Grant and Dowling, 1996) of the glutamate-elicited current, suggesting the receptor is distinct from glutamate transporters. At the photoreceptor terminals, the glutamate-elicited chloride current may regulate membrane potential and subsequent voltage-gated channel activity (i.e., Picaud et al., 1995a). Postsynaptically, this receptor is believed to mediate conductance changes underlying photoreceptor input to ONcone bipolar cells (Grant and Dowling, 1995). 7. Localization of glutamate receptor types in the retina. Fig. 13. The types of neurons in the vertebrate retina (39 K jpeg image) Photoreceptor, bipolar, ganglion cells comprise the vertical transduction pathway in the retina. This pathway is modulated by lateral inputs from horizontal cells in the distal retina and amacrine cells in the proximal retina (Fig. 13). As described in the previous sections, photoreceptor, bipolar, and ganglion cells show glutamate immunoreactivity. Glutamate responses have been electrically characterized in horizontal and bipolar cells, which are postsynaptic to photoreceptors, and in amacrine and ganglion cells, which are postsynaptic to bipolar cells. Taken together, these results suggest glutamate is the neurotransmitter released by neurons in the vertical pathway. Recent in situ hybridization and immunocytochemical studies have localized the expression of iGluR subunits, mGluRs, and glutamate transporter proteins in the retina. These findings are summarized below. 8. Retinal neurons expressing ionotropic glutamate receptors. Fig.14. Whole-cell currents in OFF bipolar cells (59 K jpeg image) Fig. 15. Whole-cell currents in horizontal cells (59 K jpeg image) In both higher and lower vertebrates, electrophysiological recording techniques have identified ionotropic glutamate receptors on the neurons comprising the OFF-pathway (Table 2, end of chapter). In the distal retina, OFF-bipolar cells (Fig. 14) (Euler et al., 1996, Sasaki and Kaneko, 1996, Hartveit, 1997) and horizontal cells (Fig. 15) (Yang and Wu, 1991, Zhou et al., 1993, Kriaj et al., 1994) respond to kainate, AMPA, and quisqualate application, but not NMDA nor APB. (However, NMDA receptors have been identified on catfish horizontal cells (OÕDell and Christensen, 1989, Eliasof and Jahr, 1997) and APB-induced hyperpolarizations have been reported in some fish horizontal cells (Nawy et al., 1989, Takahashi and Copenhagen, 1992, Furukawa et al., 1997)). Non-NMDA agonists also stimulate both amacrine cells (Fig. 16a) (Massey and Miller, 1988, Dixon and Copenhagen, 1992, Boos et al., 1993) and ganglion cells (Fig. 16b) (Mittman et al., 1990, Diamond and Copenhagen, 1993, Hensley et al., 1993, Cohen and Miller, 1994, Yu and Miller, 1995). Ganglion cells responses to NMDA have been observed (Massey and Miller, 1988, 1990, Mittman et al., 1990, Diamond and Copenhagen, 1993, Cohen and Miller, 1994); whereas, NMDA responses have been recorded in only some types of amacrine cells (Massey and Miller, 1988, Dixon and Copenhagen, 1992, Boos et al., 1993, but see Hartveit and Veruki, 1997). Fig. 16. Glutamate receptors on amacrine and ganglion cells (39 K jpeg image) Consistent with this physiological data, antibodies to the different non-NMDA receptor subunits differentially label all retinal layers (Table 3, end of chapter; Hartveit et al., 1994, Peng et al., 1995, Hughes, 1997, Pourcho et al., 1997) and mRNAs encoding the different non-NMDA iGluR subunits are similarly expressed (Hughes et al., 1992, Hamassaki-Britto et al., 1993, Brandstatter et al., 1994). In contrast, mRNAs encoding NMDA subunits are expressed predominantly in the proximal retina, where amacrine and ganglion cells are located (INL, IPL, GCL; Table 3) (Brandstatter et al., 1994, Hartveit et al., 1994), though mRNA encoding the NR2a subunit (Hartveit et al., 1994) has been observed in the OPL and antibodies to the NR2d (Wenzel et al., 1997) and the NR1 subunits (Hughes, 1997) label rod bipolar cells. 9. Retinal neurons expressing metabotropic glutamate receptors. All metabotropic glutamate receptors, except mGluR3, have been identified in retina either through antibody staining (Peng et al., 1995, Brandstatter et al., 1996, Koulen et al., 1997, Pourcho et al., 1997) or in situ hybridization (Nakajima et al., 1993, Duvoisin et al., 1995, Hartveit et al., 1995). MGluRs are differentially expressed throughout the retina, specifically in the outer plexiform layer, inner nuclear layer, inner plexiform layer, and the ganglion cell layer (Table 4, end of chapter). Though different patterns of mGluR expression have been observed in the retina, only the APB receptor on ON-bipolar cells has been physiologically examined. 10. Retinal neurons expressing glutamate transporters. The glutamate transporters GLAST, EAAC1, and GLT-1have been identified in retina (Table 5, end of chapter). GLAST (L-glutamate/L-aspartate transporter) immunoreactivity is found in all retinal layers (Otori et al. 1994), but not in neuronal tissue. GLAST is localized to Muller cell membranes (Otori et al. 1994, Derouiche and Rauen, 1995, Rauen et al., 1996, Lehre et al., 1997). In contrast, EAAC-1 (excitatory amino acid carrier-1) antibodies do not label Muller cells or photoreceptors. EAAC-1 immunoreactivity is observed in ganglion and amacrine cells in chicken, rat, goldfish, and turtle retinas. In addition, bipolar cells positive labeled with EAAC-1 antibody in lower vertebrates and immunopositive horizontal cells were observed in rat (Schultz and Stell, 1996). GLT-1 (glutamate transporter-1) proteins have been identified in monkey (Grunert et al., 1994), rat (Rauen et al., 1996), and rabbit (Massey et al., 1997) bipolar cells. In addition, a few amacrine cells were weakly labeled with the GLT-1 antibody in rat (Rauen et al., 1996), as were photoreceptor terminals in rabbit (Massey et al., 1997). 11. Summary and conclusions. Fig. 17. The ribbon glutamatergic synapse in the retina (39 K jpeg image) Histological analyses of presynaptic neurons and physiological recordings from postsynaptic cells suggest photoreceptor, bipolar, and ganglion cells release glutamate as their neurotransmitter. Multiple glutamate receptor types are present in the retina. These receptors are pharmacologically distinct and differentially distributed. IGluRs directly gate ion channels and mediate rapid synaptic transmission through either kainate/AMPA or NMDA receptors. Glutamate binding onto iGluRs opens cation channels, depolarizing the postsynaptic cell membrane. Neurons within the OFF-pathway (horizontal cells, OFF-bipolar cells, amacrine cells, and ganglion cells) express functional iGluRs. MGluRs are coupled to G-proteins. Glutamate binding onto mGluRs can have a variety of effects depending on the second messenger cascade to which the receptor is coupled. The APB receptor, found on ON-bipolar cell dendrites, is coupled to the synthesis of cGMP. At these receptors, glutamate decreases cGMP formation leading to the closure of ion channels. Glutamate transporters, found on glial and photoreceptor cells, are also present at glutamatergic synapses (Fig. 17). Transporters remove excess glutamate from the synaptic cleft to prevent neurotoxicity. Thus, postsynaptic responses to glutamate are determined by the distribution of receptors and transporters at a glutamatergic synapses which, in retina, determine the conductance mechanisms underlying visual information processing within the ON- and OFF-pathways. 12. References. Abe, T., Sugihara, H., Nawa, H., Shigemoto, R., Mizuno, N., and Nakanishi, S. (1992) Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca+2 signal transduction. J. Biol. Chem., 267, 13361-13368. Aizenman, E., Frosch, M.P., and Lipton, S.A. (1988) Responses mediated by excitatory amino acid receptors in solitary retinal ganglion cells from rat. J. Physiol., 396, 75-91. Arriza, J.L., Eliasof, S., Kavanaugh, M.P., and Amara, S.G. (1997) Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc. Natl. Acad. Sci., 94, 4155-4160. Barbour, B., Brew, H., and Attwell, D. (1988) Electrogenic glutamate uptake in glial cells is activated by intracellular potassium. Nature, 335, 433-435. Barbour, B., Brew, H., and Attwell, D. (1991) Electrogenic uptake of glutamate and aspartate into glial cells isolated from the salamander (Ambystoma) retina. J. Physiol., 436, 169-193. Bettler, B., Boulter, J., Hermans-Borgmeyer, I., OÕShea-Greenfield, A., Deneris, E.S., Moll, C., Borgmeyer, U., Hollmann, M., and Heinemann, S. (1990) Cloning of a novel glutamate receptor subunit, GluR5: expression in the nervous system during development. Neuron, 5, 583-595. Bettler, B., Egebjerg, J., Sharma, G., Pecht, G., Hermans-Borgmeyer, I., Moll, C., Stevens, C.F., and Heinemann, S. (1992) Cloning of a putative glutamate receptor: a low affinity kainate-binding subunit. Neuron, 8, 257-265. Boos, R., Schneider, H., and Wassle, H. (1993) Voltage- and transmitter-gated currents of AII amacrine cells in a slice preparation of the rat retina. J. Neurosci., 13, 2874-2888. Boulter, J., Hollmann, M., OÕShea-Greenfield, A., Hartley, M., Deneris, E., Maron, C., and Heinemann, S. (1990) Molecular cloning and functional expression of glutamate receptor subunit genes. Science, 249, 1033-1037. Bouvier, M., Szatkowski, M., Amato, A., and Attwell, D. (1992) The glial cell glutamate uptake carrier countertransports pH-changing ions. Nature, 360, 471-474. Brandstatter, J.H., Hartveit, E., Sasso-Pognetto, M., and Wassle, H. (1994) Expression of NMDA and high-affinity kainate receptor subunit mRNAs in the adult rat retina. Eur. J. Neurosci., 6, 1100-1112. Brandstatter, J.H., Koulen, P., Kuhn, R., van der Putten, H., and Wassle, H. (1996) Compartmental localization of a metabotropic glutamate receptor (mGluR7): two different active sites at a retinal synapse. J. Neurosci., 16, 4749-4756. Brew, H. and Attwell, D. (1987) Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. Nature, 327, 707-709. Cohen, E.D. and Miller, R.F. (1994) The role of NMDA and non-NMDA excitatory amino acid receptors in the functional organization of primate retinal ganglion cells. Vis. Neurosci., 11, 317-332. Derouiche, A. and Rauen, T. (1995) Coincidence of L-glutamate/L-aspartate transporter (GLAST) and glutamine synthetase (GS) immunoreactions in retinal glia: evidence for coupling of GLAST and GS in transmitter clearance. J. Neurosci. Res., 42, 131-143. Diamond, J.A. and Copenhagen, D.R. (1993) The contribution of NMDA and non-NMDA receptors to the light-evoked input-output characteristics of retinal ganglion cells. Neuron, 11, 725-738. Dixon, D.B. and Copenhagen, D.R. (1992) Two types of glutamate receptors differentially excite amacrine cells in the tiger salamander retina. J. Physiol., 449, 589-606. Duvoisin, R.M., Zhang, C., and Ramonell, K. (1995). A novel metabotropic glutamate receptor expressed in the retina and olfactory bulb. J. Neurosci., 15, 3075-3083. Egebjerg, J., Bettler, B., Hermans-Borgmeyer, I., and Heinemann, S. (1991) Cloning of a cDNA for a glutamate receptor subunit activated by kainate but not AMPA. Nature, 351, 745-748. Ehinger, B., Ottersen, O.P., Storm-Mathisen, J., and Dowling, J.E. (1988) Bipolar cells in the turtle retina are strongly immunoreactive for glutamate. Proc. Natl. Acad. Sci., 85, 8321-8325. Eliasof, S. and Jahr, C.E. (1996) Retinal glial cell glutamate transporter is coupled to an anionic conductance. Proc. Natl. Acad. Sci., 93, 4153-4158. Eliasof, S. and Jahr, C.E. (1997) Rapid AMPA receptor desensitization in catfish cone horizontal cells. Vis. Neurosci., 14, 13-18. Eliasof, S. and Werblin, F. (1993) Characterization of the glutamate transporter in retinal cones of the tiger salamander. J. Neurosci., 13, 402-411. Erulkar, S.D. (1994) Chemically mediated synaptic transmission: an overview, pp. 181-208 In Basic Neurochemistry, 5th ed. Siegel, G.J., Agranoff, B.J., Albers, R.W., and Molinoff, P.B. (eds). Raven Press, New York. Euler, T., Schneider, H., and Wassle, H. (1996) Glutamate responses of bipolar cells in a slice preparation of the rat retina. J. Neurophysiol., 16, 2934-2994. Fairman, W.A., Vandengerg, R.J., Arriza, J.L., Kavanaugh, M.P., and Amara, S.G. (1995) An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature, 375, 599-603. Furukawa, T., Yamada, K.M., Petruv, R., Djamgoz, M.B.A., and Yasui, S. (1997) Nitric oxide, 2-amino-4-phosphobutyric acid and light/dark adaptation modulate short-wavelength-sensitive synaptic transmission to retinal horizontal cells. Neurosci. Res., 27, 65-74. Fykse, E.M. and Fonnum, F. (1996) Amino acid neurotransmission: dynamics of vesicular uptake. Neurochem. Res., 21, 1053-1060. Gilbertson, T.A., Scobey, R., and Wilson, M. (1991) Permeation of calcium ions through non-NMDA glutamate channels in retinal bipolar cells. Science, 251, 1613-1615. Grant, G.B. and Dowling, J.E. (1995) A glutamate-activated chloride current in cone-driven ON bipolar cells of the white perch retina. J. Neurosci., 15, 3852-3862. Grant, G.B. and Dowling, J.E. (1996) ON bipolar cell responses in the teleost retina are generated by two distinct mechanisms. J. Neurophysiol., 76, 3842-3849. Grant, G.B. and Werblin, F.S. (1996) A glutamate-elicited chloride current with transporter-like properties in rod photoreceptors of the tiger salamander. Vis. Neurosci., 13, 135-144. Grunert, U., Martin, P.R., and Wassle, H. (1994) Immunocytochemical analysis of bipolar cells in the Macaque monkey retina. J. Comp. Neurol., 348, 607-627. Hamassaki-Britto, D.E., Hermans-Borgmeyer, I., Heinemann, S., and Hughes, T.E. (1993) Expression of glutamate receptor genes in the mammalian retina: the localization of GluR1 through GluR7 mRNAs. J. Neurosci., 13, 1888-1898. Hartveit, E. (1997) Functional organization of cone bipolar cells in the rat retina. J. Neurophysiol., 77, 1716-1730. Hartveit, E., Brandstatter, J.H., Sasso-Pognetto, M., Laurie, D.J., Seeburgh, P.H., and Wassle, H. (1994) Localization and developmental expression of the NMDA receptor subunit NR2A in the mammalian retina. J. Comp. Neurol., 348, 570-582. Hartveit, E., Brandsttter, J.H., Enz, R., and Wassle, H. (1995) Expression of the mRNA of seven metabotropic glutamate receptors (mGluR1 to 7) in the rat retina. An in situ hybridization study on tissue section and isolated cells. Eur. J. Neurosci., 7, 1472-1483. Hartveit, E. and Veruki, M.L. (1997) AII amacrine cells express functional NMDA receptors. Neuroreport,8, 1219-1223. Hensley, S.H., Yang, X.-L., and Wu, S.M. (1993) Identification of glutamate receptor subtypes mediating inputs to bipolar cells and ganglion cells in the tiger salamander retina. J. Neurophysiol., 69, 2099-2107. Hertz, L. (1979) Functional interactions between neurons and astrocytes I. Turnover and metabolism of putative amino acid transmitters. Progr. Neurobiol., 13, 277-323. Hirano, A.A. and MacLeish, P.R. (1991) Glutamate and 2-amino-4-phosphobutyric acid evoke an increase in potassium conductance in retinal bipolar cells. Proc. Natl. Acad. Sci., 88, 805-809. Hollmann, M., OÕShea-Greenfield, A., Rogers, S.W., and Heinemann, S. (1989) Cloning by functional expression of a member of the glutamate receptor family. Nature, 342, 643-648. Houamed, K.M., Kuijper, J.L., Gilbert, T.L., Haldeman, B.A., OÕHara, P.J., Mulvihill, E.R., Almers, W., and Hagen, F.S. (1991) Cloning, expression, and gene structure of a G protein-coupled glutamate receptor from rat brain. Science, 252, 1318-1321. Hughes, T.E. (1997) Are there ionotropic glutamate receptors on the rod bipolar cell of the mouse retina? Vis. Neurosci., 14, 103-109. Hughes, T.E., Hermans-Borgmeyer, I., and Heinemann, S. (1992) Differential expression of glutamate receptor genes (GluR1-5) in the rat retina. Vis. Neurosci., 8, 49-55. Ikeda, K., Nagasawa, M., Mori, H., Araki, K., Sakimura, K., Watanabe, M., Inoue, Y., and Mishina, M. (1992) Cloning and expression of the ,4 subunit of the NMDA receptor channel. FEBS Lett., 313, 34-38. Ishii, T., Moriyoshi, K., Sugihara, H., Sakurada, K., Kadotani, H., Yokoi, M., Akazawa, C., Shigemoto, R., Mizuno, N., Masu, M., and Nakanishi, S. (1993) Molecular characterization of the family of N-methyl-D-aspartate receptor subunits. J. Biol. Chem., 268, 2836-2843. Jahr, C.E. and Lester, R.A. (1992) Synaptic excitation mediated by glutamate-gated ion channels. Curr. Opin. Neurobiol., 2, 270-274. Jin, X.T. and Brunken, W.J. (1996) A differential effect of APB on ON- and OFF-center ganglion cells in the dark adapted rabbit retina. Brain Res., 708, 191-196. Johnson, J.W. and Ascher, P. (1987) Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature, 325, 529-531. Jojich, L. and Pourcho, R.G. (1996) Glutamate immunoreactivity in the cat retina: a Quantitative study. Vis. Neurosci., 13, 117-133. Kalloniatis, M. and Fletcher, E.L. (1993) Immunocytochemical localization of the amino acid neurotransmitters in the chicken retina. J. Comp. Neurol., 336, 174-193. Kanai, Y. and Hediger, M.A. (1992) Primary structure and functional characterization of a high-affinity glutamate transporter. Nature, 360, 467-471. Kanai, Y., Smith, C.P., and Hediger, M.A. (1994) A new family of neurotransmitter transporters: the high-affinity glutamate transporters. FASEB J., 8, 1450-1459. Kanai, Y., Trotti, D., Nussberger, S., and Hediger, M.A. 1997. The high-affinity glutamate transporter family, structure, function, and physiological relevance, pp. 171-213. In M.E.A. Reith (ed) Neurotransmitter transporters: structure, function, and regulation, Humana Press, Totowa, NJ. Kandel, E.R., Schwartz, J.H., and Jessell, T.M. (1991) Principles of neuroscience, 3rd ed. Elsevier Publishing Co, New York. Katsuwada, T., Kashiwabuchi, N., Mori, H., Sakimura, K., Kushiya, E., Araki, K., Megure, H., Masaki, H., Kumanishi, T., Arakawa, M., and Mishina, M. (1992) Molecular diversity of the NMDA receptor channel. Nature, 358, 36-41. Keinanen, K., Wisden, W., Sommer, B., Werner, P., Herb, A., Versoorn, T.A., Sakmann, B., and Seeburg, P.H. (1990) A family of AMPA-selective glutamate receptors. Science, 249, 556-560. Kessler, M., Arai, A., Quan, A., and Lynch, G. (1996) Effect of cyclothiazide on binding properties of AMPA-type glutamate receptors: lack of competition between cyclothiazide and GYKI 52466. Molecular Pharmacology, 49, 123-131. Kittila, C.A. and Massey, S.C. (1995) Effect of ON pathway blockade on directional selectively in the rabbit retina. J. Neurophysiol., 73, 703-712. Kleckner, N.W. and Dingledine, R. (1988) Requirement for glycine activation of NMDA-receptors expressed in Xenopus oocytes. Science, 241, 835-837. Knopfel,T., Kuhn, R., and Allgeier, H. (1995) Metabotropic glutamate receptors: novel targets for drug development. J. Med Chem., 38, 1417-1426. Koulen, P., Kuhn, R., Wassle, H., and Brandstatter, J.H. (1997) Group I metabotropic glutamate receptors mGluR1" and mGluR5a: localization in both synaptic layers of the rat retina. J. Neurosci., 17, 2200-2211. Kriaj, D., Akopian, A., and Witkovsky, P. (1994) The effects of L-glutamate, AMPA, quisqualate, and kainate on retinal horizontal cells depend on adaptational state: implications for rod-cone interactions. J. Neurosci., 14, 5661-5671. Lehre, K.P., Davanger, S., and Danbolt, N.C. (1997) Localization of the glutamate transporter protein GLAST in rat retina. Brain Res., 744, 129-137. Lodge, D. (1997) Subtypes of glutamate receptors. Historical perspectives on their pharmacological differentiation, pp. 138 In The ionotropic glutamate receptors, Monaghan, D.T. and Weinhold, R.J. (eds), Humana Press, New Jersey. Marc, R.E. and Lam, D.M.K. (1981) Uptake of aspartic and glutamic acid by photoreceptors in goldfish retina. Proc. Natl. Acad. Sci., 78, 7185-7189. Marc, R.E, Liu, W.-L.S., Kalloniatis, M., Raiguel, S.F., and Van Haesendonck, E. (1990) Patterns of glutamate immunoreactivity in the goldfish retina. J. Neurosci., 10, 4006-4034. Massey, S.C. (1990) Cell types using glutamate as a neurotransmitter in the vertebrate retina. Progr. Retinal Res., 9, 399425. Massey, S.C., Koomen, J.M., Liu, S., Lehre, K.P., and Danbolt, N.C. (1997) Distribution of the glutamate transporter GLT1 in the rabbit retina. Invest. Ophthal. Vis. Sci., 38, S689. Massey, S.C. and Miller, R.F. (1988) Glutamate receptors of ganglion cells in the rabbit retina: evidence for glutamate as a bipolar cell transmitter. J. Physiol., 405, 635-655. Massey, S.C. and Miller, R.F. (1990) N-Methyl-D-Aspartate receptors of ganglion cells in rabbit retina. J. Neurophysiol., 63, 16-30. Massey, S.C., Redburn, D.A., and Crawford, M.L.J. (1983) The effects of 2-amino-4-phosphobutyric acid (APB) on the ERG and ganglion cell discharge of rabbit retina. Vision Res., 23, 1607-1613. Masu, M., Tanabe, Y., Tsuchida, K., Shigemoto, R., and Nakanishi, S. (1991) Sequence and expression of a metabotropic glutamate receptor. Nature, 349, 760-765. Masu, M., Iwakabe, H., Tagawa, Y., Miyoshi, T., Yamashita, M., Fukuda, Y., Sasaki, H., Hiroi, K., Nakamura, Y., Shigemoto, R., Takada, M., Nakamura, K., Makao, K., Katsuki, M., and Nakanishi, S. (1995) Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell, 80, 757-765. Mayer, M.L. and Westbrook, G.L. (1987) Permeation and block of N-Methyl-D-Aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J. Physiol., 394, 501-527. Meguro, H., Mori, H., Araki, K., Kushiya, E., Kutsuwada, T., Yamazaki, M., Kumanishi, T., Arakawa, M., Sakimura, K., and Mishina, M. (1992) Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature, 357, 70-74. Mittman, S., Taylor, W.R., and Copenhagen, D.R. (1990) Concomitant activation of two types of glutamate receptor mediates excitation of salamander retinal ganglion cells. J. Physiol., 428, 175-197. Moyner, H., Sprengel, R., Schoepfer, R., Herb, A., Higuchi, M., Lorneli, H., Burnashev, N., Sakmann, B., and Seeburg, P.H. (1992) Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science, 256, 1217-1221. Moriyoshi, K., Masu, M., Ishii, T., Shigemoto, R., Mizuno, N., and Nakanishi, S. (1991) Molecular cloning and characterization of the rat NMDA receptor. Nature, 354, 31-37. Naito, S. and Ueda, T. (1983) Adenosine triphosphate-dependent uptake of glutamate into Protein I-associated synaptic vesicles. J. Biol. Chem., 258, 696-699. Nakajima, Y., Iwakabe, H., Akazawa, C., Nawa, H., Shigemoto, R., Mizuno, N., and Nakanishi, S. (1993) Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectively for L-2-amino4-phosphobutyrate. J. Biol. Chem., 268, 11868-11873. Nakanishi, S. (1992) Molecular diversity of glutamate receptors and implications for brain function. Science, 258, 597-603. Nakanishi, S. (1994) Metabotropic glutamate receptors: synaptic transmission, modulation, and plasticity. Neuron, 13, 1031-1037. Nakanishi, N., Schneider, N.A., and Axel, R. (1990) A family of glutamate receptor genes: evidence for the formation of heteromultimeric receptors with distinct channel properties. Neuron, 5, 569-581. Nawy, S. and Copenhagen, D.R. (1987) Multiple classes of glutamate receptor on depolarizing bipolar cells in retina. Nature, 325, 56-58. Nawy, S. and Copenhagen, D.R. (1990) Intracellular cesium separates two glutamate conductances in retinal bipolar cells of goldfish. Vision Res., 30, 967-972. Nawy, S. and Jahr, C.E. (1990) Suppression by glutamate of cGMP-activated conductance in retinal bipolar cells. Nature, 346, 269-271. Nawy, S.E., Sie, A., and Copenhagen, D.R. (1989) The glutamate analog 2-amino-4-phosphobutyrate antagonizes synaptic transmission from cones to horizontal cells in the goldfish retina. Proc. Natl. Acad. Sci., 86, 1726-1730. Nestler, E.J. and Duman, E.S. (1994) G proteins and cyclic nucleotides in the nervous system, pp. 429-448 In Basic Neurochemistry, 5th ed. Siegel, G.J., Agranoff, B.W., Albers, R.W., and Molinoff, P.B. (eds). Raven Press, New York. Nomura, A., Shigemoto, R., Nakamura, Y., Okamoto, N., Mizuno, N., and Nakanishi, S. (1994) Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat bipolar cells. Cell, 77, 361-369. Nowak, L., Bregestovski, P., Ascher, P., Herbet, A., and Prochiantz, A. (1984) Magnesium gates glutamate-activated channels in mouse central neurones. Nature, 307, 462-465. OÕDell, T.J. and Christensen, B.N. (1989) Horizontal cells isolated from catfish retina contain two types of excitatory amino acid receptors. J. Neurophysiol., 61, 1097-1109. Otori, Y., Shimada, S., Tanaka, T., Ishimoto, I., Tana, Y. and Tohyama, M. (1994) Marked increase in glutamate-aspartate transporter (GLAST/GluT-1) mRNA following transient retinal ischemia. Mol. Brain Res., 27, 310-314. Ozawa, S. and Rossier, J. (1996) Molecular basis for functional differences of AMPA-subtype glutamate receptors. News Physiol Soc., 11, 77-82. Peng, Y.W., Blackstone, C.D., Huganir, R.L., and Yau, K.W. (1995) Distribution of glutamate receptor subtypes in the vertebrate retina. Neurosci., 66, 483-497. Picaud, S., Larsson, H.P., Wellis, D.P., Lecar, H., and Werblin, F. (1995a). Cone photoreceptors respond to their own glutamate release in the tiger salamander. Proc. Natl. Acad. Sci., 92, 9417-9421. Picaud, S.A., Larsson, H.P., Grant, G.B., Lecar, H., and Werblin, F.S. (1995b) Glutamate-gated chloride channel with glutamate-transporter-like properties in cone photoreceptors of the tiger salamander. J. Neurophysiol., 74, 1760-1771. Pin, J.P. and Bockaert, J. (1995) Get receptive to metabotropic glutamate receptors. Curr. Opin. Neurobiol., 5, 342-349. Pin, J.P. and Duvoisin, R. (1995) Review: Neurotransmitter receptors I: The metabotropic glutamate receptors: structure and functions. Neuropharmacol., 34, 1-26. Pines, G., Danbolt, N.C., Bjoras, M., Zhang, Y., Bendahan, A., Eide, L., Koepsell, H., Storm-Mathisen, J., Seeberg, E., and Kanner, B.I. (1992) Cloning and expression of a rat brain L-glutamate transporter. Nature, 360, 464-467. Pourcho, R.G., Cai, W., and Qin, P. (1997) Glutamate receptor subunits in cat retina: light and electron microscopic observations. Invest. Ophthal. Vis. Sci., 38, S46. Pow, D.V. and Crook, D.R. (1996) Direct immunocytochemical evidence for the transfer of glutamine from glial cells to neurons: use of specific antibodies directed against the D-stereoisomers of glutamate and glutamine. Neurosci., 70, 295-302. Ransom, R.W. and Stec, N.L. (1988) Cooperative modulation of [3H}MK-801 binding to the N-Methyl-D-Aspartate receptor-ion channel complex by L-glutamate, glycine, and polyamines. J. Neurochem., 51, 830-836. Rauen, T., Rothstein, J.F., and Wassle, H. (1996) Differential expression of three glutamate transporter subtypes in the rat retina. Cell Tissue Res., 286, 325-336. Sasaki, T. and Kaneko, A. (1996) L-glutamate-induced responses in OFF-type bipolar cells of the cat retina. Vision Res., 36, 787-795. Saugstad, J.A., Kinzie, J.M., Mulvihill, E.R., Segerson, T.P., and Westbrook, G.L. (1994) Cloning and expression of a new member of the L-2-amino-4-phosphobutyric acid-sensitive class of metabotropic glutamate receptors. Mol. Pharmacol., 45, 367-372. Schultz, K. and Stell, W.K. (1996) Immunocytochemical localization of the high-affinity glutamate transporter, EAAC1, in the retina of representative vertebrate species. Neurosci. Lett., 211, 191-194. Schwartz, E.A. and Tachibana, M. (1990) Electrophysiology of glutamate and sodium co-transport in a glial cell of the salamander retina. J. Physiol., 426, 43-80. Slaughter, M.M. and Miller, R.F. (1981) 2-amino-4-phosphobutyric acid: a new pharmacological tool for retina research. Science, 211, 182-184. Slaughter, M.M. and Miller, R.F. (1983) The role of excitatory amino acid transmitters in the mudpuppy retina: an analysis with kainic acid and N-methyl aspartate. J. Neurosci., 3, 1701-1711. Stryer, L. (1988) Biochemistry, 3rd edition. W.H. Freeman and Co. New York. Tabb, J.S. and Ueda, T. (1991) Phylogenetic studies on the synaptic vesicle glutamate transporter. J. Neurosci., 11, 18221828. Tachibana, M. and Kaneko, A. (1988) L-glutamate-induced depolarization in solitary photoreceptors: a process that may contribute to the interaction between photoreceptors in situ. Proc. Natl. Acad. Sci., 85, 5315-5319. Takahashi, K.-I. and Copenhagen, D.R. (1992) APB suppresses synaptic input to retinal horizontal cells in fish: a direct action on horizontal cells modulated by intracellular pH. J. Neurophysiol., 67, 1633-1642. Tanabe, Y., Masu, M., Ishii, T., Shigemoto, R., and Nakanishi, S. (1992) A family of metabotropic glutamate receptors. Neuron, 8, 169-179. Taylor, W.R. and Wassle, H. (1995) Receptive field properties of starburst cholinergic amacrine cells in the rabbit retina. Eur. J. Neurosci., 7, 2308-2321. Van Haesendonck, E. and Missotten, L. (1990) Glutamate-like immunoreactivity in the retina of a marine teleost, the dragonet. Neurosci. Lett., 111, 281-286. Verdoorn, T.A., Burnashev, N., Monyer, H., Seeburg, P.H., and Sakmann, B. (1991) Structural determinants of ion flow through recombinant glutamate receptor channels. Science, 252, 1715-1718. Wenzel, A., Benke, D., Mohler, H., and Fritschy, J.-M. (1997) N-methyl-D-aspartate receptors containing the NR2D subunit in the retina are selectively expressed in rod bipolar cells. Neuroscience, 78, 1105-1112. Westbrook, G.L. and Mayer, M.L. (1987) Micromolar concentrations of Zn+2 antagonize NMDA and GABA responses of hippocampal neurons. Nature, 328, 640-643. Williams, K., Zappia, A.M., Pritchett, D.B., Shen, Y.M., and Molinoff, P.B. (1994) Sensitivity of the N-Methyl-D-Aspartate receptor to polyamines is controlled by NR2 subunits. Mol. Pharmacol., 45, 803-809. Yamada, K.A. and Tang, C.-M. (1993) Benzothiadiazides inhibit rapid glutamate receptor desensitization and enhance glutamatergic synaptic currents. J. Neurosci., 13, 3904-3915. Yang, C.-Y. (1996) Glutamate immunoreactivity in the tiger salamander retina differentiates between GABA immunoreactive and glycine-immunoreactive amacrine cells. J. Neurocytol., 25, 391-403. Yang, C.-Y. and Yazulla, S. (1994). Glutamate-, GABA-, and GAD-immunoreactivities co-localize in bipolar cells of tiger salamander retina. Vis. Neurosci., 11, 1193-1203. Yang, J.H. and Wu, S.M. (1997) Characterization of glutamate transporter function in the tiger salamander retina. Vision Res.,37, 827-838. Yang, X.-L. and Wu, S.M. (1991) Coexistence and function of glutamate receptor subtypes in the horizontal cells of the tiger salamander retina. Vis. Neurosci., 7, 377-382. Yazulla, S. (1986) GABAergic neurons in the retina. Progr. Retinal Res., 5, 1-52. Yu, W. and Miller, R.F. (1995) NBQX, an improved non-NMDA antagonist studied in retinal ganglion cells. Brain Res., 692, 190-194. Zhou, Z.J., Fain, G.L., and Dowling, J.E. (1993) The excitatory and inhibitory amino acid receptors on horizontal cells isolated from the white perch retina. J. Neurophysiol., 70, 8-19. Table 1 Metabotropic glutamate receptor groups (from Pin and Duvoisin, 1995). group mGluR agonist(s) intracellular pathway I mGluR1, mGluR5 quisqualate, ACPD increase phospholipase C activity, increase cAMP levels, increase protein kinase A activity II mGluR2, mGluR3 L-CCG-1, ACPD decrease cAMP levels III mGluR4, mGluR6. mGluR7, mGluR8 L-AP4 (APB) decrease cAMP or cGMP levels Table 2 Glutamate receptor types on retinal neurons, electrophysiological measurements. retinal cell type non-NMDA receptor NMDA receptor mGluR photoreceptors OFF-bipolar cells ON-bipolar cells references ++ (cones) Eliasof & Werblin, salamander 1993; Picaud et al., 1995b ++ (rods) salamander Grant & Werblin, 1996 ++ mudpuppy Slaughter & Miller, 1981, 1983 ++ cat Sasaki & Kaneko, 1996 ++ salamander Hensley et al., 1993 ++ rat Euler et al., 1996 ++ mudpuppy Slaughter & Miller, 1983 mudpuppy Slaughter & Miller, 1981, 1983 white perch Grant & Dowling, 1995, 1996 ++(APB) salamander Hirano & MacLeish, 1991 ++(L-AP4) salamander Hensley et al., 1993 ++(AP-4) rat ++(APB and cGMP) salamander Nawy & Jahr, 1990 ++(APB and cGMP) cat de la Villa et al., (1995) white perch Zhou et al., 1993 mudpuppy Slaughter & Miller, 1983 ++ ++(APB) ++(APB) horizontal cells ++ ++ ++ ++ amacrine cells Glutamate receptor with transporter-like species pharmacology salamander ++ ++(AII) ++ ++ ++ Euler et al., 1996 Yang & Wu, 1991 catfish O'Dell & Christensen, 1989; Eliasof & Jahr, 1997 rat Boos et al., 1993 mudpuppy Slaughter & Miller, 1983 ganglion cells ++ ++ rabbit Massey & Miller, 1988 ++ ++ rat Hartveit & Veruki, 1997 ++ (transient ++ & sustained (transient AC) AC) salamander Dixon & Copenhagen, 1992 ++ ++ Diamond & Copenhagen, 1993; salamander Mittman et al., 1990; Hensley et al., 1993 ++ ++ primates Cohen & Miller, 1994 ++ ++ rat Aizenman et al., 1988 ++ ++ mudpuppy Slaughter & Miller, 1983 ++ ++ cat Cohen et al., 1994 ++ ++ rabbit Massey & Miller, 1988; 1990 Table 3 Ionotropic glutamate receptor expression in retinal neurons and retinal layers, immunocytochemistry and in situ hybridization. retinal cell type or layer non-NMDA receptor subunits photoreceptors OPL NMDA receptor subunits species references GluR6/7 (single cone outer segments) goldfish Peng et al., 1995 GluR1 (cone pedicles) cat Pourcho et al., 1997 GluR2, GluR2/3, GluR6/7 rat Peng et al., 1995 cat Hartveit et al., 1994 GluR2, GluR2/3 (photoreceptors) goldfish Peng et al., 1995 GluR2 (Mb cells) goldfish Peng et al., 1995 GluR2, GluR2/3 rat Peng et al., 1995 NR2D (RBC) rat Wenzel et al., 1997 NR1 (RBC) rat Hughes, 1997 rat Hughes et al., 1992 NR2A (punctate) bipolar cells GluR2 and/or GluR4 GluR2 (RBC) horizontal cells INL GluR6/7 goldfish Peng et al., 1995 GluR2/3 cat Pourcho et al., 1997 GluR2/3, GluR6/7 rat Peng et al., 1995 rat Hartveit et al., 1994 GluR1, 2, 5 > GluR4 (outer third), GluR1, 2, 5 (middle third), GluR1-5 (inner third) rat Hughes et al. 1992 GluR1-7 rat, cat HamassakiBritto et al., 1993 NR2A(inner) NR1 (homogenous), NR2A-B KA2 (homogenous), GluR6 (inner third, patchy), NR2C rat (inner), GluR7 (inner two thirds) (inner two-thirds) Brandstatter et al., 1994 GluR1, GluR2/3, GluR6/7 rat Peng et al., 1995 NR2A rat, cat, rabbit, monkey Hartveit et al., 1994 NR2A-C rat Brandstatter et al., 1994 GluR2/3 cat Pourcho et al., 1997 GluR1, GluR2/3 rat Peng et al., 1995 ganglion cells GluR1 rat Peng et al., 1995 GCL GluR2/3, GluR6/7 rat Peng et al., 1995 GluR1-5 rat Hughes et al., 1992 GluR1-7 rat, cat HamassakiBritto et al., 1993 rat Brandstatter et al., 1994 rat Peng et al., 1995 IPL amacrine cells GluR6 GluR6-7, KA2 Muller cells GluR4 NR1, NR2A-C Table 4 Metabotropic glutamate receptor expression in retinal neurons and retinal layers, immunocytochemistry and in situ hybridization. retinal cell type Group 1 or layer OPL Group II mGluR1alpha, mGluR5a (RBC dendrites) Koulen et al., 1997 mGluR6 (RBC dendrites) rat Nomura et al., 1994 mGluR8 mouse Duvoisin et al., 1995 mGluR6 rat Nakajima et al., 1993 mGluR6 (RBC), mGluR7 (BC), mGluR4,7 (AC) rat Hartveit et al., 1995 rat Peng et al., 1995 rat Brandstatter et al., 1996 mGluR1alpha, mGluR5a (AC dendrites) rat Koulen et al., 1997 mGluR1alpha rat Peng et al., 1995 cat Pourcho et al., 1997 rat Peng et al., 1995 mouse Duvoisin et al., 1995 cat Pourcho et al., 1997 rat Hartveit et al., 1995 mGluR5 (BC, HC), mGluR1 (AC) mGluR2 (AC) mGluR1alpha mGluR7 (CBC terminals; AC dendrites; few GC dendrites) amacrine cells mGluR1alpha ganglion cells species references rat INL IPL Group III mGluR2/3 mGluR1alpha GCL mGluR8 mGluR1alpha mGluR2/3 mGluR1 mGluR2 mGluR4, 7 Table 5 Glutamate transporters in retinal neurons and retinal layers, immunocytochemical localizations. retinal cell type EAACGLAST GLT-1 1 + (cone soma to pedicles) photoreceptors OPL ++ species reference rabbit Massey et al., 1997 rat Rauen et al., 1996 ++ (rod spherules > rabbit cone pedicles) rat Schultz & Stell, 1996; Rauen et al., 1996 ++ (2 types of CBCs) rabbit Massey et al., 1997 ++ rat Rauen et al., 1996 turtle, salamander Schultz & Stell, 1996 ++ (DB2, flat midget bipolar cells) monkey Grunert et al., 1994 ++ (diffuse) rabbit Massey et al., 1997 ++ rat Rauen et al., 1996 goldfish, salamander, turtle, chicken, rat Schultz & Stell, 1996 rat Rauen et al., 1996 horizontal cells ++ bipolar cells ++ (faint) ++ IPL ++ ++ amacrine cells ++ ++ ++ ganglion cells Muller cells Massey et al., 1997 Schultz & Stell, 1996 ++ chicken, rat, goldfish, turtle Schultz & Stell, 1996 ++ rat Rauen et al., 1996 rat Rauen et al., 1996; Lehre et al., 1997; Deroiche & Rauen, 1995 ++ Color Vision by Peter Gouras 1. Introduction. Fig. 1. Geometric drawing with 15 or more colors (59 K jpeg image) Fig. 2. Geometric drawing in black and white and shades of gray (59 K jpeg image) Color vision is known best by man's perception of it. It creates a unique dimension to sight that is impossible to appreciate by any non-visual means. It depends on wavelength more than on the energy of light but it is an illusion of reality resulting from a comparison of the responses of nerve cells in our brain. Color and all vision are in a sense illusory depending only on messages that pass between millions of neurons that reside within the darkness of our skull. These visual messages allow us to project ourselves into a universe that would be unknown to us without vision. Much is known about human color vision both subjectively and quantitatively from the fields of physics, psychology and physiology. Physiology attempts to explain color vision by the responses of neurons. This is the ultimate step in understanding color and eventually perhaps in constructing machines that will see a similar universe of colors. Fig. 3. The visual pathways from retina to visual cortex of the human brain (98 K jpeg image) Linking subjective human experience with single neural responses, however, has many pitfalls. Most of the information comes from electrophysiological recordings from neurons at the peripheral levels of vision, the retina and the lateral geniculate nucleus of monkeys, while subjective human experience involves the entire brain (Fig. 3). There are vast areas of the visual brain, especially cerebral cortex, that remain relatively uncharted because they are so unresponsive in anesthetized monkeys. But it is only in these centers that the subjective experience of color occurs. Therefore, we have to use our imagination to extrapolate from the early stages in vision at the retinal level to explain the physiology of color vision at higher levels in our brain. This is where the major difficulty lies. It will ultimately be resolved by new techniques that allow examination of the awake, perceiving human brain. I start by presenting my ideas on how human color vision has evolved from a primative divariant system and go on to consider the transmission of hue, form and contrast in parallel streams from the retina to the cerebral cortex. I suggest neural circuitry underlying the perception of color in the retina, lateral geniculate and striate cortex and beyond. 2. The Evolution of Vision Vision must have started with organisms detecting the difference between light and dark. Molecular genetics reveals that this began in broad daylight with cone photoreceptors (Bowmaker, 1998), a hypothesis suggested earlier by the comparative anatomist, Gordon Walls (1942). Under these conditions shadows must have been the main stimuli for detecting movements and objects. A shadow depolarizes, i.e. activates, a cone, which releases a neurotransmitter that depolarizes second order retinal neurons, bipolar cells, called off-center bipolars and horizontal cells. The reappearance of light by movement hyperpolarizes the cones. This stops the release of the transmitter, which in turn stops, i.e. hyperpolarizes, the off-bipolars and horizontal cells. This also disinhibits a parallel system of bipolars, called on-bipolars. Disinhibition occurs because the same transmitter released by the cone inhibits the on- and excites the off-bipolars. Onand off- cone bipolars have different receptors for the same transmitter (see chapter on the Outer plexiform layer). This push-pull arrangement of on and off -bipolars (Fig. 4) provides the main input to the brain about the visual universe. One channel signals darkness; the other signals lightness. Fig. 4. The push-pull arrangement of on- and off-bipolar cells (59 K jpeg image) In order for cones to function under the enormous changes in ambient light in sunlit scenes, they must regulate their sensitivity. This has evolved within the biochemistry underlying phototransduction (Fig. 5). Light absorbed by a protein (opsin from the Greek word to see) coupled to the 11-cis isomer of retinaldehyde activates the opsin, which in turn activates another protein, transducin. This in turn activates an enzyme, phosphodiesterase that breaks down a cyclic nucleotide to close the cone's membrane to depolarizing ions. This chain of reactions amplifies the light signal. A shadow reverses this process opening the membrane to depolarizing ions. The brighter the ambient light, the briefer is the phototransduction reaction and the faster is it reversed by a shadow. This increased speed of the amplification process reduces sensitivity but quickens the response. This is an important part of visual adaptation, which allows cones to function over a large range of light energy. In addition, there is also negative feedback mediated by horizontal cells that antagonize the responses of cones. Fig. 5. The phototransduction cascade in rod or cone outer segments (59 K jpeg image) In a world seen by only one type of cone, objects appear lighter or darker but not in color. Whether an object is light or dark depends on a comparison between the light energy it reflects and that reflected by its background. The brighter the background, the darker the object appears. The darker the background, the lighter the object appears. Absolute energy is sacrificed for relative energy, which is all that is necessary for survival. 3. Color Vision Inhabiting a world in which an organism can only distinguish light and dark without color is a handicap. Therefore early in the evolution of vision, color must have appeared. For this, two different types of cones were necessary, one responding best to one part and a second responding best to another part of the visible spectrum, i.e. sunlight. By this means the brain can compare two signals to distinguish color. Again the brain senses relative rather than absolute differences, in this case of wavelengths rather than energies of light. Fig. 6. The wavelength sensitivities of the different photoreceptor types in the vertebrate retina. Blue-violet and yellowgreen wavelengths maximally stimulate the two cone types in the divariant mammalian retina (59 K jpeg image) A second cone system evolved that was most sensitive to the short wavelength region of the visible spectrum, the region we call bluish-violet (Fig. 6). The output of these cones could be compared with the earlier long wave cones that evolved to detect light and dark, and are most sensitive to the yellow-green part of the spectrum (Fig. 6). The spectral sensitivity of a cone is determined by the absorption spectrum of its opsin. The further apart these absorption spectra are, the greater is the potential color contrast (see later Fig. 12). Why not use a red rather than a yellow-green sensitive opsin? There are diminishing returns in using red opsins, probably due to their lower quantal energies. Why not use an ultra-violet opsin? Ultra-violet light is strongly absorbed by the cornea and lens before it reaches the retina and exhibits much chromatic aberration. If these structures are comparatively small as in mice, an ultra-violet cone becomes more tractable. With two different spectral images of an object and/or its background, the brain can derive differences that are impossible to detect with only one spectral image. Now the brain can distinguish objects, which are not just lighter or darker than their background but have as new attribute, color. An object is uniquely white, gray or black if the two different cone mechanisms are affected equally by the reflected energy of the object and its background. If an inequality exists between the two cone mechanisms, detecting the light reflected by either the object or its background, the brain will see color, making the object or its background appear different than white, gray or black. Objects can have the same brightness as their backgrounds but stand out because of this inequality. This is a powerful way to see objects in a world where most things reflect about the same light as their background. Nature uses these two cone mechanisms differently. The long wave cones are the sole determinant of light and dark. The short wave cones are only used for color contrast. This strategy minimizes chromatic aberration. For this reason there are about ten times as many long than short wave sensitive cones in most retinas. In the human central fovea there are very few short wave cones and borders are detected by brightness alone. 4. Chromatic versus Achromatic Contrast To distinguish light and dark as well as color, two parallel neural circuits are used in visual cortex. One depends only on long wave cones; it detects differences that depend on light energy. This system is responsible for distinguishing light and dark, i.e. achromatic contrast. The second depends on both long and short wave cones; it compares the response of both cone systems to the same stimulus. This system is responsible for chromatic contrast. Together both systems are responsible for color vision. To distinguish light from dark, neighboring groups of long wave cones are compared for borders of energy contrast; these borders are assembled in the brain as an object (Fig. 7, left). This comparison can involve single neighboring cones or rows of cones at the minimum angle of visual resolution. In the fovea this is a micron, about 1/200th of a degree. Fig. 7. Neural circuits for Achromatic contrast and Chromatic contrast (59 K jpeg image) For chromatic contrast, the brain compares the responses of a group of long wave cones with a group of short wave cones in the same retinal area by taking the difference between them (Fig. 7, right). It would be ambiguous to compare only one cone with a neighboring cone because a difference between the two could be caused by energy rather than wavelength gradients. In order to prevent this ambiguity, groups of cones are compared. Such a group of cones can be considered a unit area of chromatic space to distinguish it from a unit area of achromatic space, which in the fovea is a single cone. Therefore achromatic space is more finely divided than chromatic space (Mullen, 1985). For spatial contrast, the long and short wave cone difference is compared with a difference signal derived from neighboring groups of cones. This creates borders of chromatic (wavelength) contrast. The brain combines borders of chromatic and achromatic contrast to detect objects. 5. Divariant Mammalian Color Vision About 2% of human males have only two types of cones; long and short wave sensitive. This is a two-variable, i.e. divariant, color vision system similar to many other mammals, such as dogs and cats. Both the achromatic and chromatic contrast signals are transmitted by long wave cones via the same channels of bipolar and ganglion cells (Fig. 8, left). Small, slowly conducting bipolar and ganglion cells transmit tonic signals of the long wave cones to the brain. One channel is excited by an increment of light absorption by long wave cones; this is an on-channel. Another channel is excited by a decrement of light absorption by long wave cones; this is an off-channel. In the brain both signals are processed by two separate circuits, one of which extracts achromatic and the other chromatic contrast. For achromatic contrast, signals of neighboring tonic long wave cone channels are compared. For chromatic contrast signals from groups of long wave cone channels are compared with those of short wave cone channels. Fig. 8. Neural circuits for the tonic signals of the long wave cones (59 K jpeg image) The short wave cones transmit their signals by another tonic system of bipolar and ganglion cells (Fig. 8, right). In this case there are only short wave cone on-channels, presumably because these cones are only involved in chromatic and not in achromatic contrast. In addition these ganglion cells receive and excitatory input from long wave cone off-bipolars. This increases their sensitivity to successive chromatic contrast, i.e. short wave following long wave light. For chromatic contrast the brain makes two comparisons. It compares the short and long wave cone signals in the same retinal area and then compares this with neighboring areas to detect borders of chromatic contrast. There is a separate fast, phasic system of larger bipolars and ganglion cells that also transmits signals from long wave cones to the brain (Fig. 9). This system is not as highly developed in the fovea as the tonic system and has a lower spatial resolution. It appears to play no role in color vision. It has a high sensitivity to achromatic contrast (Kaplan and Shapley, 1986) and is sensitive to slow movements (unpublished results). Evidence exists that it contributes to luminance (Lee et al, 1990). Fig. 9. Neural circuits for the phasic signals of the long wave cones (59 K jpeg image) 6. Simultaneous Contrast An object's achromatic contrast and brightness depends entirely on the difference between the light it reflects and the light reflected by its surround. Color also depends on simultaneous contrast of wavelength rather than energy contrast. Edwin Land demonstrated the importance of simultaneous contrast in color vision by showing that color depends on the light reflected from the surround of an object. He proposed a model in which the comparison of one cone system with another, needed to arrive at a decision for color, occurs after an object's brightness has been established separately for each cone mechanism. He used the term lightness to describe the brightness of such a monochrome (monocone) image (Land, 1986). Fig.10. The retinex model of Edwin Land (59 K jpeg image) Figure 10 illustrates the reasoning behind his retinex (retino-cortex) model. Two projectors cast light on a screen. One transmits white light; the other transmits only long wavelength light. A shadow (arrow) is cast on the screen by blocking the long wave projector (Fig. 10A left). The illumination seen by the two cone systems is shown on the right. The short wave cones see 100% of the white light but 0% of the long wave light. The long wave system sees 100% of the white light and 100% of the long wave light except in the area of the shadow where it sees about 1% of the scattered light. When both projectors are on, the long wave cones detect about 101% of the light from the shadow and 200% from its surround. Therefore the shadow is relatively dark to the long wave cones (Fig. 10A). The retinal area of the shadow seen by the short wave cones absorbs the same light as its surround; therefore it is not dark to these cones. Because the shadow is darker to the long than the short wave cone system, it has a bluish color. This occurs even though more light energy is absorbed from the shadow by the long than the short wave cones. It is the relative long wavelength gradient of the shadow that determines its color. This decrease in the lightness image of the long wave cones compared to the short wave cones makes the shadow of the arrow, blue (Fig. 10B). Land's algorithm, which computes the lightness of an object for each cone mechanism before comparing these values for color predicts the appearance of colors in a natural scene (Vitek, 1997). Accordingly, the comparison of the short and long wave cone systems, necessary for color vision, should occur after the normalization for lightness has been established within each cone system. This requires independent processing of the tonic long and short wave cone images before they are compared. In divariant subjects, the long wave cone system is absolutely independent of the short wave cones and therefore satisfies this requirement (Fig.8). The short wave cone system transmits a signal that is relatively independent of the long wave cone system (Fig. 8). It receives input from long wave cone offbipolars but this only augments its response to short wave light. How are these separate cone images normalized? Figure 11 provides a reasonable scheme. It is based on so called doubleopponent neurons, found in the visual cortex of primates (Michael, 1977). Double-opponent neurons have spatial antagonism between the same cone mechanism mediating the center and surround of their receptive field, something not seen strongly at the level of the retina or geniculate. In this model, long wave cones, responding to the object, excite the neuron and long wave cones, responding to its background, inhibit the neuron. This inhibition normalizes the responses over space because it creates a difference between the responses of different retinal areas, eliminating absolute values (Ratliff, 1960). After normalization chromatic contrast is achieved by two comparisons. One occurs between neurons reflecting the lightness images of these two cone systems in the same area of space, antagonistically interacting with each other to create neurons responsive to the difference between the two. A second comparison involves this difference signal being compared with those of neighboring areas of visual space to detect simultaneous chromatic contrast. Fig.11A shows how a neuron responding to yellow is formed. B. How a neurons responding to blue is formed. C. System of neurons responding to white and D. system of neurons responding to black (59 K jpeg image) Figure 11A suggests how a neuron responding to yellow could be formed. It receives an excitatory, normalized input from the long wave cone on- system and an inhibitory input from a normalized short wave on-system. Figure 11B shows a neuron that responds only to blue. It receives an excitatory, normalized input from the short-wave on-system and a normalized, inhibitory input from the long wave cone on-system. The long and the short wave cone systems are compared by subtraction. One difference creates a neuron that is excited by ³yellow²(Fig. 11A). The converse creates a neuron excited by blue (Fig. 11B). These neurons are most sensitive to simultaneous color contrast, the former to yellow on a blue background, the latter to blue on a yellow background. These responses resist changes in the spectral composition of the illuminant, i.e. they show color constancy. They are sensitive to wavelength rather than energy contrast. White, gray or black is seen when there is no chromatic (wavelength) contrast, i.e. as in a black and white photograph (Fig. 2). The yellow and blue neurons are silent. Another set of neurons is responding. Pure white requires both the long wave and the short wave cone systems to be responding equally. Therefore the long wave on- and the short wave on-systems must both be excited. Black requires both of these systems to be silent and the long wave cone off-system to be excited. There must be separate systems of neurons responding to white (Fig. 11C) and black (Fig. 11D). If both are excited simultaneously, the sensation must be gray. 7. Trivariant Human Color Vision Normal human color vision depends on three not two cone mechanisms. This adds an additional dimension to color vision creating reds and greens. To do this nature splits the long wave system into two similar systems with slightly different spectral sensitivities with relatively similar opsins (Fig. 12) (Nathans et al., 1986) but the same neural machinery duplicated in parallel circuits. One cone opsin is most sensitive to yellow-green and the other to yellow-red. This splits the brightest and yellow part of the visible spectrum into two color bands, one green and the other red. This red-green system works in parallel with that for blue-yellow. Fig.12. The closely related molecular structure of the cone opsins. The blue-cone opsin compared with rhodopsin. The blue cone opsin compared with the green opsin and the minimal difference between the red and green cone opsins. The pink filled circles represent amino acid substitutions between these molecules. The open circles indicate identical amino acids. Adapted from Nathans et al., 1986 (59 K jpeg image) The splitting of the long wave cone channel into two creates four longer wave channels in the tonic system, two for reddishyellow and two for greenish-yellow sensitive on- and off-signals (Fig. 13A). The phasic system, which plays no role in color vision mixes both cones in the same on- or off-bipolar cell and retains its original two channels (Figure 13B). In visual cortex, the signals from the tonic reddish-yellow or long wave sensitive (L-) cone and greenish-yellow or middle wave sensitive (M-) cone channels are again used to detect achromatic (energy) and chromatic (wavelength) contrast (Fig. 14A and B). Fig. 13. The different circuits for Tonic and Phasic pathways through the retina (59 K jpeg image) Fig. 14. The signals from the tonic reddish-yellow or long wave sensitive (L-) cone and greenish-yellow or middle wave sensitive (M-) cone channels are used to detect A. achromatic and B. chromatic (wavelength) contrast in the cortex (59 K jpeg image) Trivariance allows the mixing of these parallel red-green and blue-yellow systems to produce non-spectral colors of magenta (red and blue) or cyan (green and blue) (Fig. 15). Fig.15. Trivariance of the primate fovea compared with divariance of the normal mammalian retina (59 K jpeg image) Trivariance introduces a second comparison of cone signals, in this case between the two long wave cone systems to create red-green contrast. It occurs in parallel with the divariant comparison of short and long wave cones; in this case both long wave cones are compared to the short wave cones for blue-yellow contrast. If there is a significant difference between the signals of the two long wave cone systems, there will be a hue difference of red or green. If there is no difference between the signals of the two long wave cone systems, the detector will default to the divariant comparison. If there is no difference for the divariant comparison, the signal will appear white, gray or black. It is important to realize that the absorption spectrum of the reddish-yellow cone opsin is not only more sensitive to long wave (red) light but also more sensitive to short wavelength (violet) light than the greenish-yellow cone opsin This gives a reddish color to short wavelengths. 8. Hue, Saturation and Brightness Color is complex because it depends on more than wavelength contrasts alone (Kaiser & Boynton, 1996). This was appreciated in the 19th century by psychologists who defined the terms hue, saturation and brightness to define color. Hue defines the wavelength contrast aspect of color, such as yellow or blue and red or green. Saturation defines the mixing of hue with white, gray or black. A saturated color has strong hue with little or no white, i.e. blood red. An unsaturated color has its hue washed away by white, i.e. pink. It is reasonable to assume that the independent responsiveness of parallel circuits is responsible for the mixing of hue with white, black or gray. A third quality of color is brightness, which is less obvious than the other two. Yellow and white tend to be bright and blue and black tend to be dark. This difference appears to track the on- and off-systems of the long wave cones. Whenever the on-system is responsive, yellows and whites are likely to be perceived and they tend to be bright. Whenever the off-system is active, blacks and blues are likely to be perceived and they tend to be dark. In addition other qualities of the image such as texture or gloss contribute to color as in gold and silver. The Opponent Color Theory of the 19th century physiologist Ewald Hering (Hering, 1964; Hurvich, 1981) derived by the analysis of subjective human color vision is in general correct, although the idea of opponent colors was described earlier by Goethe and Schoepenhauer. Certain colors are not perceived together, i.e. they do not mix. We never see bluish-yellows or reddish-greens. This is consonant with the neural comparisons described previously. The yellow detector is always inactive when the blue detector is active and vice versa. A similar situation occurs for the neurons responding to red or green. Hering's theory was brilliant but it was proposed when little was known about the anatomy and physiology of the retina. Hering and his school (Ladd-Franklin, 1929) considered that the antagonism between colors occurred in the retina. We now know that color vision is established not in the retina but in visual cortex. The arrangement resembles stereoscopic vision, which is also established in visual cortex, where common signals from each eye are used by different neural circuits to sample objects at different distances in space. Early recordings of the responses of single neurons in primate retina and geniculate nucleus revealed cells excited by red and inhibited by green light or vice versa (Fig. 17) (Wiesel and Hubel, 1966; Gouras, 1968). These were thought to be the red/green opponent color channel of Hering. Cells were also detected that were excited by blue and inhibited by yellow light or the converse (Fig. 17). These were thought to be the blue/yellow channel of Hering. In addition there were cells which were excited or inhibited by all wavelengths. These were thought to be Hering's white/black channel (DeValois et al., 1966). Now almost half a century later, this view appears to be a misconception. We shall here consider why. 10. Hering Red-Green Channel in the Retina The cells excited by red and inhibited by green light or the converse, which were considered to be Hering's red-green channel, are comprised of four different types of cells. Two sets are on- and two sets are off-center cells. One set has L-cone on- or off- centers and another has M-cone on- or off-centers. The cells with L-cone centers receive antagonistic signals from M-cones in the surround of their receptive field. The cells with M-cone centers receive antagonistic signals from Lcones in the surround of their receptive field. The strength of this cone-cone antagonism varies considerably. Fig. 17. Color opponent receptive field structure of primate tonic ganglion cells (59 K jpeg image) Fig. 18. Electrophysiological recordings from color and spatially opponent midget ganglion cell in the monkey retina (59 K jpeg image) The first problem with these cells being Hering's red-green opponent channel is that the opponency depends on the geometry of stimulation. Large spots resemble an opponent channel but small spots centered on their receptive field do not, i.e. there is no opponency. The antagonistic surround signal requires more spatial summation than the center mechanism (Fig. 18). A second problem is that a cell with a green sensitive off-center is supposed to be contributing to the sensation of red as much as a cell with a red sensitive on-center. These two cells, which are logically transmitting opposite signals about local brightness, seem inappropriately labeled as a single Hering red-green channel. A third problem arises when one compares the excitation and inhibition these cells receive from the opposing cone mechanisms. Only a fraction are actually inhibited by red and excited by green light or the converse. Many are excited more by yellow or white light than other colors, which is not really a Hering red-green channel. Fig.19. The wiring of red and green color and spatially opponent midget ganglion cells of the monkey fovea (59 K jpeg image) There is additional complexity to the L- and M- tonic cone system. It is generally agreed that these cells comprise the midget ganglion cells of the fovea. These retinal ganglion cells receive a direct input from a midget bipolar cell, which receives its input from a single L- or M-cone. The cone antagonism is thought to come indirectly through horizontal and amacrine cells (Fig. 19). There is evidence that the cone antagonism mediated by horizontal cells comes from both L- and M-cones (Dacey, 1996), which is also inconsistent with a Hering red-green opponent channel. A rival view discounts any role of midget ganglion cells in color vision (Rodieck, 1998; Dacey, 1996). This hypothesis rests on evidence of the existence of a small number of geniculate cells that have coextensive receptive fields with L-cones exciting and M-cones inhibiting or the converse (Wiesel & Hubel, 1966). This result has been difficult to confirm. These cells lack the geometry problem mentioned previously because the red-green opponency does not vary with stimulus size. Coextensive L- and M-cone opponent cells have also been reported to be in the intercalated layers (koniocellular layers) of the geniculate and to project to the areas of visual cortex where double opponent color cells are located (see later figures 25, 26 and 27). These results also need confirmation. Some support for this hypothesis comes from recordings in the peripheral retina of midget-like ganglion cells, where Land M- cones are synergistic rather than antagonistic (Dacey and Lee, 1997). This result has been taken to imply that the midget system plays no role in color vision. Another interpretation is that the antagonistic organization of midget ganglion cells is lost outside the fovea because peripheral 'midgets' are not truly midget ganglion cells at all: they receive more than one midget bipolar input and multiple cone inputs (Kolb et al., 1998). So trivariance, may be lost in the peripheral retina. I believe that all L- and M- tonic cells contribute to color vision but they are not the red-green opponent channels of Hering. I suggest that the antagonism these cells show between L-and M- cone mechanisms is a spectral filter, narrowing the spectral band to which they respond best. This resembles oil droplets located in the cones of certain diurnal vertebrates like birds, reptiles (Fig. 20) and fishes. Oil droplets narrow the action spectrum of the cones, increasing color contrast. Long wave cones become longer wave selective; middle wavelength cones become more middle wavelength selective. This phenomenon makes the monochrome retinex image even more monochrome. Fig. 20. Wholemount view of the turtle retina showing the characteristic oil droplets within the different spectral types of cones (59 K jpeg image) 11. Hering Blue-Yellow Channel in the Retina Some cells excited by blue and inhibited by yellow light or the converse were considered to be Hering's blue-yellow channel. This view is also being challenged nowadays. The first problem is that most of these cells are excited by light stimulation of short wave cones, either as blue or white light. The Hering blue-yellow channel should not be responsive to white light. The excitation these cells receive from L- and M- off bipolars contributes to successive contrast but exerts little inhibition on the short wave cone signals. A second problem is their polarity. There are many more excited than inhibited by short wave cones. Malpeli and Schiller (1978) and Gouras and Zrenner (1981) have concluded that retinal and geniculate cells inhibited by short wave cones do not exist. This view is contested by Lee et al. (1987) who have tried carefully to detect cells inhibited by short wave cones. This is not an easy task because cells in the tonic L-and M-cone system can be inhibited by blue and excited by yellow light. In order to eliminate this possibility, Lee et al. (1987) used stimuli, which changed along a tritanopic axis of color space, to which only short wave cones respond. They found cells inhibited by this stimulus. However, macular pigments or other factors could cause their stimuli to miss the tritanopic axis of primate color space thus weakening their conclusion. Fig. 21. Intracellular responses of the blue-yellow ganglion cell of the primate retina (Dacey and Lee., 1994) (59 K jpeg image) Anatomy has revealed that the short wave cone excitatory signal uses a unique bistratified retinal ganglion cell organized to be excited by short wave cones absorbing light and excited by off-responses of longer wave cones (Fig. 21); the converse has not been found. The three dimensional structure of the short wave cone synaptic pedicle reveals a synaptic organization that resembles rod spherules more than longer wave cone pedicles (see chapter on S-cone pathways). The Scone bipolar dendrites have contacts with S-cones resembling those of on-bipolars of rods. The existence of short wave cone specific off-bipolars is therefore questionable (Kolb et al , 1997). Electroretinography also suggests that in contrast to the L- and M-cones, there is no evidence of a short wave cone offbipolar response (Evers & Gouras, 1986). The short wave on-channel in primate retina is briefly inhibited when a long wave field is turned off, making a blue flash transiently disappear to an observer. This phenomenon was discovered psychophysically by Stiles (1949) and confirmed by Mollon and Polden (1977). This phenomenon is also seen in retinal ganglion cells mediating the excitatory signal of short wave cones (Gouras, 1968), providing a unique psychological marker for the human short wave cone system. A yellow flash does not disappear when a blue adapting field is turned off, which might be expected if there were symmetrical short wave inhibitory-long wave excitory cells. This too supports the absence of a short wave cone off-channel. Short wave cones have little impact on brightness but have a strong influence on color. If there were short wave cone offbipolars, they should be logically related to signaling darkness. This would conflict with the L- and M-cones, which signal darkness when their off-bipolar system goes off. In the presence of yellow light the short wave cone off-bipolars would go off when the long wave cone on-bipolars go on. On this reasoning short wave cone off- channels are inappropriate. 12. Hering White-Black Channel in the Retina Although these sensations are opposite in nature, they are not opponent as blue and yellow or red and green colors are. The intermediary sensation of gray is a mixture of black and white. The reason for this is that no antagonism occurs between cone mechanisms for the establishment of white and black. These sensations depend on antagonism in space but not among cones. It is not a wavelength but an energy comparison. White or gray depends on all cone mechanisms absorbing light and for pure white or gray this absorption rate must be relatively equal. If the short wave channel is not active, the whites and grays become yellowish, greenish or reddish. The early recordings of single retinal and geniculate neurons in monkeys revealed a subset of cells that were either excited or inhibited by all wavelengths. These were considered to represent the white and black channels of Hering. At that time, there was no distinction between tonic (parvo) and phasic (magno) systems. The idea that these cells reflect the perception of white and black is incorrect because they respond to all colors, not just white or black. The perception of white and black occurs in the visual cortex, where simultaneous contrast occurs. I suggest that pure white occurs when the normalized images of the three cone on-systems are equal for the object. Pure black occurs when the normalized L-and Mcone off -systems are equally excited and the short wave cone on-system is silent in the representation of the object in the cortex. 13. Retinal Interneurons The horizontal and amacrine cells represent laterally interacting elements in the retina. In general they are inhibitory (antagonistic) in their neural interactions. There are exceptions, notably the rod amacrine cell, which transmits rod signals to bipolars and ganglion cells (see chapter on circuitry for rod signals). Among primate horizontal cells there are at least two major classes. One class receives its input overwhelmingly from Land M-cones. A second class receives its input from all three cones but much more from short wave ones (Fig. 22) (Ahnelt and Kolb, 1994; Dacey, 1996). It is thought that horizontal cells feedback antagonistically on cones. The specificity of the feedback is not clearly defined. It is possible that the horizontal cells receiving inputs from short wave cones only exert feedback on to short wave cones, even though they receive inputs from L- and M-cones. I favor this hypothesis because one does not see any input from short wave cones in ganglion cells receiving inputs from L- and M-cones. If the horizontal cells receiving inputs from short wave cones were to feedback on to L- and M-cones, short wave cone inputs would be found in all retinal ganglion cells, which is not the case. Fig. 22. The cone and rod inputs to the three horizontal cell types of the primate retina (Ahnelt and Kolb, 1994) (39 K jpeg image) What is the role of horizontal cells? It would seem that the negative feedback they exert on the cones is to control overdriving of the cones by large energy gradients in both space and time. Over-stimulation of the cones would be curtailed and response speed increased. Spatial contrast could also be facilitated (Ratliff, 1960). The horizontal cell feedback is only brought into action by strong stimuli that affect large groups of neighboring cones. This could also enhance spectral contrast. The depolarization that yellow light would exert on S-cones through horizontal cells could enhance any subsequent hyperpolarization produced by short wave light. This would augment responses to short wave light on long wave backgrounds and facilitate successive color contrast. The amacrine cells are less understood than horizontal cells. It is reasonable to assume that most transmit antagonistic interactions to bipolar and ganglion cells. The amacrine cell interaction occurs after the on- and off- systems of bipolar cells are established. This allows separate channels of antagonism to be mediated by on- and off- amacrine cells. Their role would be similar to horizontal cells but at the inner plexiform layer (Fig. 23). Fig. 23. The midget bipolar to ganglion cell pathways and proposed horizontal and amacrine cells that could form antagonistic surrounds in the primate retina (39 K jpeg image) Another role of the amacrine cell system is to help establish the functional differences in the tonic and phasic ganglion cell systems. The complete circuitry of these two systems is not known. It is likely that the phasic system has separate on- and off- bipolars that transmit signals from cones to the ganglion cell layer (Fig.13) because midget cone bipolars synapse only on midget ganglion cells and not on other ganglion cells (Kolb, 1994; Boycott and Wassle,1999). Therefore, another system of L- and M-cone bipolars, presumably diffuse cone bipolar types, (see chapter on cone pathways through the retina) must transmit cone signals to phasic ganglion cells. It would be at these bipolars and/or ganglion cells that amacrine cells establish the phasicity of the phasic ganglion cells (Werblin, 1991; Slaughter et al., 1995; Cook and McReynolds, 1998). Land M-cone signals reach these ganglion cells faster than they reach the tonic ganglion cells (Gouras, 1968), which also suggests a separate bipolar system. 14. The Role of Phasic Ganglion Cells The first recordings from cells in the magno-cellular layers of the lateral geniculate of monkeys were by Wiesel and Hubel (1966). The sample of cells they encountered in these layers was relatively small. They described cells that were tonically inhibited by red light, a phenomenon not found in their more extensive sampling of the parvo-cellular layers. Two different classes of ganglion cells occur next to each other in monkey retina, one phasic, the other tonic (Gouras, 1968; DeMonasterio and Gouras, 1975). These differences are striking in the retina where these two different types of cells can be recorded from simultaneously. Antidromic driving showed that phasic cells have faster conduction velocities than tonic ones and therefore were presumably larger cells, suggesting a link with the magno- and parvo-cellular layers of the geniculate, respectively. The antidromic field potential of these two cell groups revealed that the tonic cell system was concentrated around the fovea while the phasic system was evident perifoveally and peripherally (Gouras, 1969). Fig. 24. Electrophysiological recordings from phasic ganglion cells in the monkey perifovea (DeMonasterio and Gouras, 1975) (59 K jpeg image) It is difficult to know what visual sensation the phasic system mediates. It has been suggested that it is responsible for luminance (Lee et al., 1990). Luminance is an additive quality, which has an action spectrum reflecting the combined L-and M- cone systems but not short wave cones. It has also been suggested that the phasic system detects movement. One of the reasons for this hypothesis is that the phasic ganglion cell system projects to the magno-cellular layers of the lateral geniculate nucleus, which in turn projects through striate cortex to a visual area MT, where cells sensitive to movement and the direction of movement seem to be found. This idea is not completely accepted. It is also possible that the phasic system mediates the signal for the optokinetic reflex, responding to very slow retinal movements (unpublished observations). The phasic system is not involved in color vision because it synergistically mixes the signals of L- and M-cones, which is not what one expects from a system involved in color vision. It is possible that the signal of the phasic system is used for blueyellow contrast. However, I doubt this hypothesis because 1) the retinal distribution of the phasic ganglion cells is not in register with that of the tonic system and 2) their latencies and conduction velocities are out of phase with those of the tonic system. Why should one visual pathway be phasic and the other tonic? This is an intriguing question that awaits more research. It has been suggested that the foveally oriented tonic pathway plays a more dominant role in conscious perception in which tonic responses may be an advantage (Martinez-Conde et al., 1999). 15. The Lateral Geniculate Nucleus The lateral geniculate nucleus (see Fig. 3) forms the main stream of visual information to the cerebral cortex. It is a transfer center that disentangles the various retinal subsystems serving the contralateral visual fields and organizes their projections to striate cortex. Fig. 25. The projections of the major ganglion cell classes of the retina to the layers of the lateral geniculate nucleus in the primate (59 K jpeg image) Fig. 26. Histological section through the primate lateral geniculate nucleus (LGN) to show the layering of the neurons into 4 parvocellular layers, 2 magnocellular layers and 6 koniocellular layers (59 K jpeg image) The tonic system, carrying cone specific channels for both high visual resolution and color vision, is confined to the parvocellular layers (Fig. 25). The phasic system is confined to the magno-cellular layers (Fig. 25). The signals from each eye are kept separate in order to combine them appropriately for stereoscopic vision, where different combinations reflect different depth planes. Similarly on-cells are separated from off-cells and cone specific responses are kept separate for color vision. There are non-retinal inputs to the lateral geniculate nucleus that must modulate the flow of information but they are poorly understood. They may play a role in attention and sleep. 16. Color Vision in Visual Cortex The cerebral cortex mediates conscious perception. In striate cortex there are local zones called blobs (Fig. 27) that contain cells that exhibit double-opponent behavior (Michael, 1977; Livingstone and Hubel, 1988). We assume double-opponency is an essential stage in color vision. Double-opponency is not introduced at an earlier stage because a commitment to color contrast cannot be made until the achromatic information is extracted from the tonic L- and M-cone input channels. Fig. 27. Diagram of a slab of striate cortex (V1) of primate brain to show the composition of a hypercolumn. A hypercolumn consists of two ocular dominace columns (one from each eye) each containing stacks of orientation columns. A blob is a cylinder of cells running from I to IVB which receives direct input from blue/yellow cells of the koniocellular layers of the LGN, and the color-opponent red and green cells of the parvocellular layers of the LGN. The latter projections are secondary to the first synapses in layer IVCb. Magnocellular cells from the LGN project to layer IVCa (59 K jpeg image) Achromatic and chromatic information is considered to be multiplexed in the tonic L- and M-cone system, which is demultiplexed before chromatic contrast is established. By demultiplexing is meant that chromatic information is transmitted to different neural circuits than those for achromatic information (Fig. 27). The L-and M-cone on- and offchannels are used synergistically for achromatic contrast detectors. These same channels are used antagonistically for chromatic contrast detectors. Achromatic contrast involves larger populations of neurons located outside the smaller blob areas (Fig. 27, achromatic and oriented). There is no multiplexing for the short wave channel because it is totally committed to color. Thus this channel goes directly from LGN to the blobs (Fig. 27). Double-opponency involving the short wave cone system also depends on the demultiplexing of the longer wave cone system's signal before a comparison is made for chromatic contrast. We assume that chromatic contrast begins with double-opponent cells (Fig. 11). Double-opponency establishes wavelength contrast independently of brightness contrast. These two forms of contrast become independent neural entities in establishing borders of contrast. Orientation selectivity, undoubtedly essential for form vision, could be based on either or both of these forms of contrast. The greater spatial resolution of achromatic contrast appears to involve more orientation selective cells in visual cortex than color contrast does. 17. Color Vision beyond Striate Cortex Knowledge of the physiology of color vision beyond striate cortex is sketchy because of the difficulty in exciting cortical cells in anesthetized monkeys. Post striate visual cortex is composed of a number of separate areas. One area, called MT, appears to receive its major input from the magno-system. Several other areas, called visual areas 2, 3 and 4 receive their input from the parvo-system. The role of these different areas is unclear (Schiller and Lee, 1991). Color selective cells have been found in areas 1, 2, 3 and 4 (Kruger and Gouras, 1979), although Zeki (1993) has maintained that they are much more numerous in visual area 4 (V4). Using three projectors to control the spectral reflectance of objects in a Mondrian-like display, Zeki (1993) reported a major difference between striate cortex and visual area 4. Spectrally selective cells in striate cortex of an anesthetized monkey responded to the energies reflected from the objects and not to their color. In other words, objects with different colors but reflecting identical energies from their surface evoked the same response when presented to such neurons. On the other hand, spectrally selective cells in area V4 responded to the colors and not to the energies. This implies that color is not established in striate cortex but at a later stage in vision. Zeki has raised the interesting hypothesis that V4 is the visual center for color and is not represented in a retinotopic but in a chromatic coordinate system. An object's shape would be defined in area V1 (striate cortex) and perhaps V2 where the anatomy reflects a retinotopic plan, and its color would be defined in V4 following a chromatic plan. In V4 colors would be distributed in an orderly way over the structure somewhat like a chromaticity diagram (Fig. 27). This is a radical view of cortical processing that needs more research. A problem with this hypothesis is how a neural response that represents an object in one cortical area can be linked with its color response in another area. Color is complex depending not only on hue, defined by the chromaticity diagram but also by achromatic cues such as shadowing and texture. Another view is that chromatic contrast detectors are ubiquitous in all visual areas, providing parallel cues to borders with achromatic contrast detectors. The remapping of visual space, occurring in these multiple visual areas may increase the universe of forms and objects distinguishable (Fig. 28). Fig. 27. The CIE chromaticity diagram which is a standard method for specifying colored lights or light reflected from materials (from Normann et al., 1991) (59 K jpeg image) Fig. 28. Proposed cortical areas and interactions between them for processing of color vision (59 K jpeg image) The Commision Internationale d'Elairage (CIE) chromaticity diagram (Figure 27) defines human trivariant color experience in a quantitative way, which allows standards for color comparisons worldwide. It is defined by three spectral variables, which can be combined to mimic any color. The values of the three standards have been normalized so that they always add up to 1.0. In this way the color diagram can be plotted in two dimensions, those of the long (red) and middle (green) wave standards. What does not equal 1.0 on the diagram represents the short wave standard. In the middle of the diagram, where the three standards are all 0.33, the color is white (gray or black). Moving up the diagram makes the color green, right makes the color red and down makes it blue. The value 1.0 can never be reached because the absorption spectra of the three cone opsins overlap so much that the standards stimulate more than one cone mechanism. Therefore, all colors are to some extent desaturated. Perhaps if one could find a way to stimulate only one class of cones, more saturated colors might be perceived. Normal humans can perceive about a million different colors but this number depends on the right conditions. The illumination must be bright and the size of the color stimulus must be large, at least 20 degrees of visual angle to appreciate such a large universe of colors. Why very large stimuli create a much greater palette of colors must depend on spatial summation in visual cortex (Figure 28). 18. Color and Form One of the most impressive facts about the physiology of visual cortex is the relatively small proportion of cells that have any striking wavelength selectivity. This is in marked contrast to the retino-geniculate pathway where many cells show wavelength selectivity. This suggests that much of the neural machinery in visual cortex is used for pattern recognition rather than color vision, and that wavelength selectivity plays only a minor role in the response repertoire of cortical visual neurons. Evidently the processing of a black and white (achromatic) image requires almost as much neural power as the processing of a color image: perhaps like a color television, where much more information is transmitted for the pattern than for the color of the image. Just where color enters into pattern recognition will require a better understanding of the latter. It would seem that objects are identified by connected groups of orientation selective neurons having appropriate contrasts (Marr, 1982) and these objects are reinforced by their structural stability with movement in space. In this, light, dark and shading have considerable impact on contour stability and structure as evidenced by the pattern recognition possible in black and white photographs. Color adds an important but minor frill to this complex neural construct but because of its limited dimensionality (only two or three dimensions), it has become an alluring femme fatale to many of us. 19. References. Ahnelt, P. and Kolb, H. (1994) Horizontal cells and cone photoreceptors in human retina: A Golgi-electron microscopic study of spectral connectivity. J. Comp. Neurol. 343, 406-427. Bowmaker, J. K. (1998) Evolution of color vision in vertebrates. Eye 12,541-547. Boycott, B. B. and Wassle, H. (1999) Parallel processing in the mammalian retina. Invest. Ophthal. Vis. Sci. 40,113-1327. Cook, P. B., and McReynolds, J. S. (1998) Lateral inhibition in the inner retina is important for spatial tuning of ganglion cells. Nature Neuroscience 1, 714-9. Dacey, D. M. (1996) Circuitry for color coding in the primate retina. Proc. Nat. Acad. Sci. 93, 582-588. Dacey, D. M. and Lee, B. B. (1997) Cone inputs to the receptive fields ofmidget ganglion cells in the periphery of Macaque retina. Invest. Ophthal. Vis. Sci 38, S708. Dacey, D. M. and Lee, B. B. (1994) The blue-on opponent pathways in primate retina originates from a distinct bistratified ganglion cell. Nature 367, 731-735. DeMonasterio F. M. and Gouras, P. (1975) Functional properties of ganglion cells of the rhesus monkey retina. J. Physiol. (Lond) 251,167-95. DeValois, R. L., Abramov, I. and Jacobs, G. H. (1966) Analysis of response patterns of LGN cells. J. Opt. Soc. Am. 56, 966977. Dowling, J. E. (1987) The Retina: an approachable part of the brain. The Belknap Press, Harvard University Press Cambridge, Massachusetts. Evers, H. and Gouras, P. (1986) Three cone mechanisms in the primate electroretinogram: two with and one without offcenter bipolar responses. Vision Res. 26, 245-254. Gouras, P. (1968) Identification of cone mechanisms in monkey ganglion cells. J. Physiol. 199, 533-547. Gouras, P. (1969) Antidromic responses of orthodromically identified ganglion cells in monkey retina. J. Physiol. 204, 407419. Gouras, P. and Zrenner E. (1981) Color vision: a review from a neurophysiological perspective. Prog. in Sens. Physiol. 1,139-179. Hering, E. (1964) Outlines of a theory of the light sense. Translated by L.M. Hurvich & D. Jameson. Cambridge, MA: Harvard University Press. Hurvich, L.M. (1981) Color Vision. Sunderland, MA: Sinauer Assoc.Inc. Kaiser, P. and Boynton, R. M. (1996) Human Color Vision. Opt. Soc. of Amer. Kaplan, E. and Shapley, R. M. (1986) The primate retina contains two groups of ganglion cells, with high and low contrast sensitivity. Proc. Natn. Acad. Sci. USA 83, 2755-2757. Kolb, H. (1994) The architecture of functional neural circuits in the vertebrate retina. Invest. Ophthal. Vis. Sci. 35, 23852404. Kolb, H., Goede, P., Roberts, S., McDermott, R., and Gouras, P. (1997) Uniqueness of the S-cone pedicle in the human retina and consequences for color processing. J. Comp. Neurol. 386, 443-460. Kolb, H., DeKorver, L., Church, J., Crooks, J., Jacoby, R. and Marshak, D. (1998) P cells of the primate retina. Invest. Ophthal. Vis. Sci. 39, S563. Kruger, J. and Gouras, P. (1979) Spectral selectivity of cells and its dependence on slit length in monkey visual cortex. J. Neurophysiol. 43, 1055-1069. Ladd-Franklin (1929) Land, E. (1986) Recent advances in retinex theory. Vision Res. 26.7-21. Lee, B. B., Pokorny, J., Smith, V. C., Martin, P. R., and Valberg, A. (1990) Luminance and chromatic modulation sensitivity of macaque ganglion cells and human observers. J. Opt. Soc. Am. 7, 2223-2236. Lee, B. B., Valberg, A., Tigwell, D. A. and Tryti, J. (1987) An account of spectrally opponent neurons in macque lateral geniculate nucleus to successive contrast. Proc. Roy. Soc. (Lond.) B 230, 293-314. Livingstone, M. S. and Hubel, D. H. (1988) Segregation of form, color, movement and depth: Anatomy, physiology and perception. Science 240, 740-749. Malpeli, J. G. and Schiller, P. (1978) Lack of blue off-center cells in the visual system of the monkey. Brain Res. 141. 385389. Marr, D. (1982) Vision. San Francisco W. H. Freeman & Co. Martinez-Conde, S., Macknik, S. L. and Hubel, D. H. (1999) Bursts of spikes in the LGN and area V-1 are correlated with microsaccades during visual fixation in the behaving monkey. Invest. Ophthalmol. Vis. Sci. 40, S642. Michael, C. (1977) Color vision mechanisms in monkey striate cortex: dual opponent cells with concentric receptive fields. J. Neurophysiol. 41, 572-588, 1987. Mollon, J. D. and Polden, P. G. (1977) An anomaly in the response of the eye to light of short wavelength. Phil. Trans. Roy. Soc. (Lond.) B 238 207-240. Mullen, K. T. (1985) The contrast sensitivity of human colour vision to red-green and blue-yellow chromatic gratings. J. Physiol. 359, 381-400. Nathans, J., Thomas, D., and Hogness, D. S. (1986) Molecular genetics of human color vision: The genes encoding blue, green and red pigments. Science 232, 203-210. Ratliff, F. (1960) Mach Bands: Quantitative studies on neural networks in the retina. Holden Day Inc. San Francisco. Rodieck, R. W. (1998) The First Steps in Seeing. Sunderland, MA: Sinauer Assoc. Schiller, P. H. and Lee, K. (1991) The role of primate area V4 in vision. Science 251,1251-1253. Schiller, P. H., Logothetis, N. K. and Charles, E. R. (1990) Functions of the the color-opponent and the broad-band channels in vision. Visual Neurosci. 5, 321-346. Schiller, P. H., Logothetis, N. K. and Charles, E. R. (1991) Parallel pathways in the visual system: their role in perception. Neuropsychologia 29, 433-442. Slaughter, M.M, Zhang, J. & Tian, N. (1995) Ramifications of GABA receptor subtypes on retinal information processing. In J. Robbins (ed): Basic and Cilinical Perspectives in Vision Research. New York: Plenum Press, pp. 115-124. Stiles, W.S. (1949) Increment thresholds and the mechanisms of colour vision. Documenta Ophth. 3,138-165. Walls, G. L. (1942) The vertebrate retina and its adaptive radiation. Cranbrook Press, Michigan. Werblin F. S. (1991) Synaptic connections, receptive fields, and patterns of activity in the tiger salamander retina. Invest. Ophthal. Vis. Sci., 32, 459-483. Wiesel, T. and Hubel, D. H. (1966) Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J. Neurophysiol. 29,1115-1156. Zeki, S. A (1993) Vision of the Brain. Oxford, Blackwell Scientific. < From: http://webvision.med.utah.edu/color.html>