* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Protein Kinase A Activation Down-Regulates, Whereas Extracellular
Organ-on-a-chip wikipedia , lookup
Tissue engineering wikipedia , lookup
Hedgehog signaling pathway wikipedia , lookup
Cell encapsulation wikipedia , lookup
List of types of proteins wikipedia , lookup
Cellular differentiation wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Purinergic signalling wikipedia , lookup
Paracrine signalling wikipedia , lookup
Cannabinoid receptor type 1 wikipedia , lookup
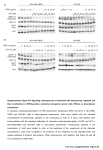
0022-3565/07/3201-202–210 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS U.S. Government work not protected by U.S. copyright JPET 320:202–210, 2007 Vol. 320, No. 1 108415/3164862 Printed in U.S.A. Protein Kinase A Activation Down-Regulates, Whereas Extracellular Signal-Regulated Kinase Activation Up-Regulates -1 Receptors in B-104 Cells: Implication for Neuroplasticity Gianfrancesco Cormaci, Tomohisa Mori, Teruo Hayashi, and Tsung-Ping Su Cellular Pathobiology Unit/Development and Plasticity Section/Cellular Neurobiology Research Branch, Intramural Research Program/National Institute on Drug Abuse/National Institutes of Health/Department of Health and Human Services, Baltimore, Maryland Received May 24, 2006; accepted October 17, 2006 The -1 receptor (Sig-1R) is a unique intracellular nonopioid binding site with nano- to low-micromolar affinities for diverse classes of drugs including benzomorphans, antidepressants, neuroleptics, steroid hormones including progesterone, and psychostimulants such as cocaine and methamphetamine (Sharkey et al., 1988; Su et al., 1988; Snyder and Largent, 1989; Quirion et al., 1992; Nguyen et al., 2005). This work was supported by the Intramural Research Program of National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services. G.C., T.M., and T.H. contributed equally to this work. Article, publication date, and citation information can be found at http://jpet.aspetjournals.org. doi:10.1124/jpet.106.108415. sulfonamide dihydrochloride (H-89). However, dB-cAMP, when used at 2 mM, caused an up-regulation of Sig-1Rs that was insensitive to the H-89 blockade but was partially blocked by an extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase inhibitor PD98059 (2⬘-amino-3⬘-methoxyflavone). Furthermore, 2 mM dB-cAMP induced an ERK phosphorylation lasting at least 90 min, at which time the phosphorylation caused by 0.5 mM dB-cAMP had already diminished. PD98059, applied 90 min after addition of 2 mM dB-cAMP, attenuated the Sig-1R up-regulation. Our results indicate that cAMP is bimodal in regulating Sig-1R expression: a downregulation via PKA and an up-regulation via ERK. Results also suggest that psychostimulants may manipulate the cAMPPKA-Sig-1R and/or the cAMP-ERK-Sig-1R pathways to achieve a neuroplasticity that favors addictive behaviors. Sig-1Rs exist in the central and peripheral nervous systems where they modulate neurotransmitter release and cell excitability via ion channels (Monnet et al., 1990; Hayashi and Su, 2001; Aydar et al., 2002; Nuwayhid and Werling, 2003). Behavioral studies implicated Sig-1Rs in higher ordered brain functions including mood and learning and memory (Maurice et al., 2001; Meunier et al., 2004). Recently, a number of studies suggested that psychostimulant-induced behaviors involve an up-regulation of Sig-1Rs. Ujike et al. (1996) demonstrated that cocaine-induced locomotor sensitization was cross-sensitized to the Sig-1R agonist 3-(3-hydroxyphenyl)-N-(1-propyl)piperidine and that the sensitization could be suppressed by a Sig-1R antagonist, ABBREVIATIONS: Sig-1R, -1 receptor; BMY 14802, ␣-(4-fluorophenyl)-4-(5-fluoro-2-pyrimidinyl)-1-piperazine; DA, dopamine; PKA, protein kinase A; ERK, extracellular signal-regulated kinase; dB-cAMP, N6,2⬘-O-dibutyryl-cAMP; NE-100, N,N-dipropyl-2-[4-methoxy-3-(2-phenylethoxy)phenyl]-ethylamine monohydrochloride; PD98059, 2⬘-amino-3⬘-methoxyflavone; FCS, fetal calf serum; DMEM, Dulbecco’s modified Eagle’s medium; MS-377, ((R)-(⫹)-1-(4-chlorophenyl)-3-[4-(2-methoxyethyl)piperazin-1-yl]methyl-2-pyrrolidinone L-tartate; H-89, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide dihydrochloride; PAGE, polyacrylamide gel electrophoresis; TBS-T, Tris-buffered saline plus 0.05% Tween 20; ANOVA, analysis of variance; SCH23390, R-(⫹)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine; SpcBIMP, Sp isomer of cyclic-5,6-dichloro-benzimidazol-riboside thiophosphate. 202 Downloaded from jpet.aspetjournals.org at ASPET Journals on August 9, 2017 ABSTRACT The -1 receptor (Sig-1R) can bind psychostimulants and was shown to be up-regulated in the brain of methamphetamine self-administering rats. Up-regulation of Sig-1Rs has been implicated in neuroplasticity. However, the mechanism(s) whereby Sig-1Rs are up-regulated by psychostimulants is unknown. Here, we employed a neuroblastoma cell line B-104, devoid of dopamine receptors and transporter, and examined the effects of psychostimulants as well as cAMP on the expression of Sig-1Rs in this cell line, with a specific goal to identify signal transduction pathway(s) that may regulate Sig-1R expression. Chronic treatments of B-104 cells with physiological concentrations of cocaine or methamphetamine failed to alter the expression of Sig-1Rs. N6,2⬘-O-Dibutyryl-cAMP (dBcAMP), when used at 0.5 mM, caused a down-regulation of Sig-1Rs that could be blocked by a protein kinase A (PKA) inhibitor, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinoline- ERK Up-Regulates -1 Receptors postsynaptic regulation of Sig-1Rs. B-104 cells were chosen in this study as a postsynaptic model because B-104 cells are presumably devoid of DA receptors, at least the D2 receptor (Severson et al., 1983). These cells thus allow us to examine whether cocaine or methamphetamine may up-regulate Sig-1R expression in a manner independent of presynaptic DA interferences. Furthermore, B-104 cells contain cAMP, thus serving as a tool for investigating cAMP-related signals that may regulate the expression of Sig-1Rs. The results are presented in this report. Materials and Methods Materials. Cocaine hydrochloride, S-(⫹)-methamphetamine hydrochloride, and (⫹)-pentazocine were obtained from National Institute on Drug Abuse (Rockville, MD). NE-100 was a kind donation from Dr. Okuyama (Taisho Pharmaceutical Co., Saitama, Japan). N6,2⬘-O-dibutyryl-cAMP and the mitogen-activated protein kinase kinase inhibitor PD98059 were purchased from Sigma (St. Louis, MO), whereas the PKA inhibitor H-89 was from Calbiochem (La Jolla, CA). The polyclonal antibody against phospho-ERK was from Cell Signaling (Beverly, MA), and the anti-ERK antibody was from Santa Cruz Inc. (Santa Cruz, CA). Culture medium, antibiotics, and fetal calf serum were from Gibco BRL (Carlsbad, CA). Antibodies directed against dopamine receptors (D1, D2, D3, D4, and D5) and dopamine transporters were purchased from Chemicon (Temecula, CA). Sig-1R Polyclonal Antibody. The polyclonal anti-Sig-1R antibody was produced by immunizing rabbits with synthetic antigenic peptides. The antigenic peptide corresponds to the second hydrophilic domain of the rat Sig-1R (52-CLDHELAFSRRLIVELRRLH69). Anti-Sig-1R IgGs were purified from serum by using affinity column chromatography. Cell Cultures and Drug Treatments. B-104 cell cultures were maintained on a 175-cm2 flask (Falcon; BD Biosciences Discovery Labware, Bedford, MA) in 10% FCS-containing Dulbecco’s modified Eagle’s medium (DMEM), with 2 mM glutamine, 50 U/ml penicillin, and 50 g/ml streptomycin until use. Cells were cultured at 37°C with 5% CO2 under saturating humidity. The medium was changed every 2 to 3 days. Cells were seeded in 12-well plates (Falcon) in 1% FCS-containing DMEM (1 ml/well). Cells were treated with drugs in the following fashion. When a test drug was required, a half-amount of the culture medium was replaced daily with a fresh medium containing the test drug. dB-cAMP, however, was prepared as a 50 or 200 mM stock solution in deionized water, 10-l aliquots of which were then directly applied to cultured cells to reach the desired final concentration. PD98059 was dissolved in dimethyl sulfoxide as a 10 mM stock solution that, when needed, was further diluted to reach final concentrations. Cocaine, methamphetamine, (⫹)-pentazocine, and dihydrexidine were dissolved in distilled H2O. Controls received the same amount of water or dimethyl sulfoxide. Western Blotting. Cells were seeded at 5 or 10 ⫻ 104 cells/well in 12-well plates. After treatments with drugs, cells were placed on ice, rapidly washed once with ice-cold phosphate-buffered saline, and then scraped into 1 ml of phosphate-buffered saline with a rubber policeman. After transferring into Eppendorf tubes, cell suspensions were centrifuged at 3000 rpm for 5 min at 4°C. The supernatants were discarded and pellets dissolved with 50 l of 2⫻ Laemmli sample buffer (100 mM Tris-HCl, pH 6.8, 20% glycerol, 4% SDS). Samples were sonicated and assayed for proteins using a BCA kit (Pierce Chemicals, Rockford, IL). After addition of -mercaptoethanol and bromphenol blue, samples were boiled for 2 min, and proteins were resolved with a 13% polyacrylamide SDS-PAGE. For Sig-1R detection, 15 g of proteins was loaded into each lane. For detection of other proteins, proteins were loaded at 25 to 30 g per lane. In Sig-1R Western blottings, gels were electroblotted onto polyvi- Downloaded from jpet.aspetjournals.org at ASPET Journals on August 9, 2017 BMY 14802. Likewise, methamphetamine-induced locomotor sensitization was also blocked by a Sig-1R antagonist, MS377 (Takahashi et al., 2000). Up-regulation of Sig-1Rs thus has been demonstrated in specific brain areas of methamphetamine- or cocaine-sensitized rats (Itzhak, 1993; Liu et al., 2005). We found that Sig-1Rs were up-regulated by 50% in the midbrain of rats self-administering methamphetamine in a response-contingent manner (Stefanski et al., 2004). Up-regulation of Sig-1Rs promotes cellular differentiation in PC-12 cells (Takebayashi, 2002) and in oligodendrocytes (Hayashi and Su, 2004), causes the reconstitution of plasma membrane lipid rafts (Hayashi and Su, 2005), and potentiates the brain-derived neurotrophic factor signaling for glutamate release (Yagasaki et al., 2006). These findings suggest that Sig-1Rs may promote addictive processes by altering the structure or morphology of neurons (Hayashi and Su, 2005). Although cocaine or methamphetamine binds with low micromolar affinity to Sig-1Rs, exactly how these psychostimulants may up-regulate the expression of Sig-1Rs is unknown. Other factors in addition to binding may also be involved. Monoaminergic systems are related to the action of methamphetamine and cocaine. Of these, the dopaminergic system plays an important role in the methamphetamine- or cocaine-induced sensitization in animals. There are two superfamilies of dopamine (DA) receptors, designated D1- and D2-like receptors. D1 and D2 receptors are coupled to Gs and Gi proteins, respectively, thereby modulating the production of cAMP. cAMP is well known to activate protein kinase A (PKA), which, in turn, controls numerous cellular functions. However, cAMP has also been demonstrated to induce the activation of the extracellular signal-regulated kinase (ERK) (Stork and Schmitt, 2002). ERK phosphorylates several transcription-regulating molecules related to cellular differentiation and apoptosis. The ERK pathway is also known to be crucial for the establishment of behavioral responses to psychostimulants (Valjent et al., 2005). Thus, the ERK activation plays a role in the relapse to cocaine self-administration (Lu et al., 2005), the retrieval of cocaine-induced conditioned place preference (Miller and Marshall, 2005; Valjent et al., 2006a), and psychostimulant-induced sensitization (Valjent et al., 2006b). However, the cellular biological processes downstream of ERK activation leading to altered neural plasticity are not totally understood. We suggested recently a link between cAMP and the regulation of Sig-1R expression. Subchronic treatment of NG108 cells with a stable cAMP analog, dB-cAMP, induced an up-regulation of Sig-1Rs in the cell (Stefanski et al., 2004). However, the exact signaling pathway downstream of cAMP leading to the Sig-1R up-regulation was not identified. The present study, therefore, was conducted to identify the potential signal transduction pathways downstream of cAMP that may participate in the regulation of Sig-1R expression. We also aimed to understand how psychostimulants may up-regulate Sig-1Rs. Cocaine and methamphetamine might up-regulate Sig-1Rs by a direct binding to Sig-1Rs. Alternatively, psychostimulants might up-regulate Sig-1Rs through the drugs’ ability to increase DA at the synaptic cleft, which subsequently interacts with postsynaptic DA receptors. Sig1Rs were also shown to be able to regulate DA release in a model system simulating a presynaptic mechanism (Ault and Werling, 2000). The present study, however, focused on the 203 204 Cormaci et al. ethanol; 50°C) before being reprobed for the total ERK. The degree of ERK phosphorylation was calculated by dividing the intensity of p-ERK by that from the total ERK. cAMP Assays. cAMP was extracted from cell lysates with 0.1 N HCl and then measured by an immunoassay method (Assay Designs; Sigma) according to the manufacturer’s instruction. Cells were stimulated for 3 to 20 min with DA receptor ligands. Samples stimulated with 1 M forskolin for 3 to 20 min served as positive controls. Statistical Analyses. Data shown in this study are expressed as mean ⫾ S.E.M. Statistical significance was analyzed by one-way analysis of variance (ANOVA) followed by Tukey test with the significance level set at p ⬍ 0.05. Results Quantitative Western Blotting of Sig-1Rs in B-104 Cells. Affinity-purified Sig-1R antibodies against rat Sig-1R (52– 69) recognized Sig-1Rs in B-104 cells as a 25-kDa band in Western blotting (Fig. 1A). B-104 cells thus constitutively express Sig-1Rs. The linear range for a quantitative detection of Sig-1Rs in the Western blotting was established by loading different amounts of proteins into each lane. As shown in Fig. 1B, Sig-1R intensities were linearly correlated with amounts of loaded proteins (r2 ⫽ 0.9866, p ⬍ 0.001) when 5 to 25 g of proteins was used in the assay. Based on these results, 15 g of protein/lane was used in this study for quantitative measurements of Sig-1Rs. Lack of Dopamine Receptors and Dopamine Transporter in B-104 Cells. As mentioned in the introduction, to exclude psychostimulant effects via dopaminergic signaling, B-104 cells, presumably devoid of DA receptors (Severson et al., 1983), were used in this study. Using Western analyses, Fig. 1. Characterization of Sig-1R expression in B104 cells. A, representative Western blotting using an affinity-purified anti-Sig-1R antibody against the rat Sig-1R (52-CLDHELAFSRRLIVELRRLH-69). B-104 cell lysates separated by 13% SDS-PAGE were immunoblotted with either normal rabbit IgG (left) or anti-Sig-1R IgG (right). Arrowhead, position of Sig-1Rs. B, quantitative detection of Sig-1R protein levels by using Western blotting. B-104 proteins ranging from 0 to 50 g per lane were loaded (x-axis). y-axis, densitometrically analyzed intensities of Sig-1R bands. Each point represents mean ⫾ S.E.M. of six samples. Note the significantly linear regression within the range of 5 to 25 g/lane. C, dopamine receptors, tyrosine hydroxylase (TH), and dopamine transporter (DAT) are below detection levels in B-104 cell (rat brain samples serve as positive controls). Total proteins from B-104 cells (100 g/lane) and rat brains (30 g/lane) were loaded for the Western blotting. D, dopamine; R, receptor. Downloaded from jpet.aspetjournals.org at ASPET Journals on August 9, 2017 nylidene difluoride membranes (Bio-Rad, Hercules, CA) in Towbeen buffer (25 mM Tris-base, 192 mM glycine) without methanol. Membranes were blocked with 10% nonfat dry milk (Bio-Rad) in Trisbuffered saline plus 0.05% Tween 20 (TBS-T) for 1 h at room temperature. Membranes were incubated for 2 h at room temperature with anti-Sig-1R polyclonal antibodies (1:1500) in TBS-T and probed with the secondary anti-rabbit IgG horseradish peroxidase conjugate for 1 h at room temperature (1:15000 dilution). Protein bands were visualized with an ECL chemiluminescence enhancer (GE Healthcare, Little Chalfont, Buckinghamshire, UK) in a Kodak Image Station 440 CF (Kodak, New Haven, CT). For phospho-ERK western blottings, membranes were blocked in TBS-T plus 5% bovine serum albumin, then incubated overnight with polyclonal anti-pERK antibodies at a 1:1000 dilution in TBS-T plus 5% bovine serum albumin. For the detection of dopamine receptors and dopamine transporter, the Wistar rat brain was used: the striatum for D1, D2, D3, D5, and dopamine transporter; and the midbrain for D4 and tyrosine hydroxylase. The brain was quickly removed after decapitation, and brain regions were dissected out on ice. Fresh tissues were homogenized with a Daunce homogenizer in 2⫻ Laemmli sample buffer (10 v/w). After sonication and centrifugation, supernatants were subjected to SDS-PAGE. Trans-blotted membranes were incubated overnight with respective specific antibodies (1:300 dilution; Chemicon). Analysis of Protein Levels. Western blotting digital images were analyzed by using the Kodak 1D Image Analysis Software. The background intensity was subtracted from the total intensity for each band. In analyzing the Sig-1R protein levels, we did not use tubulin or actin as internal controls because cAMP or methamphetamine treatments seemed to affect these proteins. Sig-1R levels were therefore calculated based on intensities of Sig-1Rs on the same film obtained from blots from various treatment conditions. Each experiment was thus repeated at least five times. For determination of the total ERK, the membrane was first probed for p-ERK, and then the same membrane was stripped of p-ERK (5% SDS, 0.5% -mercapto- ERK Up-Regulates -1 Receptors We decided therefore to repeat the cAMP experiment using NG-108 cells and established the full dose-response curve for dB-cAMP on its regulation on the expression of Sig-1Rs. Likewise, a dose-response curve was also established for dB-cAMP on its effect on the Sig-1R expression in B-104 cells. The two curves were then compared. cAMP increased Sig1Rs in NG-108 cells in a monophasic manner (Fig. 3B, upper curve). However, cAMP affected the level of Sig-1Rs in B-104 cells apparently in a biphasic manner; firstly, with a downward trend that maximized at approximately 0.5 mM cAMP, and then with an upward trend causing an approximately 175% increase of Sig-1Rs at 2 mM cAMP (Fig. 3B, lower curve). These results from the dose-response curves suggested, among other possibilities, that cAMP in B-104 cells, unlike that seen with NG-108 cells, might have activated two different intracellular pathways that act in opposite directions in affecting the expression of Sig-1Rs. Because cAMP is known to activate mainly the PKA pathway and the ERK pathway (see Introduction), we hypothesized that the activation of one of these cAMP-activated pathways, the PKA pathway or the ERK pathway, may cause an alteration of Sig-1Rs. The following experiments were thus designed to test this possibility. Firstly, we examined whether the direct activation of PKA by an Sp isomer of cyclic-5,6-dichloro-benzimidazol-riboside thiophosphate (Sp-cBIMPS) might cause an alteration of Sig1Rs. Sp-cBIMPS dose-dependently caused a down-regulation of Sig-1Rs (Fig. 3C). To further confirm the involvement of PKA in the down-regulation of Sig-1Rs, we treated the cells with a PKA inhibitor, H-89. H-89 at 200 nM has been shown to be a highly selective PKA inhibitor (Robin et al., 1998). H-89 alone (treatment time, 24 h) was able to increase Sig-1R expression in a dose-dependent manner (Fig. 3D). The H-89 effect disappeared on day 3 (Fig. 3E). In the presence of H-89, Fig. 2. Effects of cocaine (COC), methamphetamine (METH), or Sig-1R ligands on the expression of Sig-1Rs. Sig-1R levels were measured by Western blotting as specified in Fig. 1. Data represent means ⫾ S.E.M. of six to eight independent experiments. A, effects of duration of treatment (1–3 days) with cocaine or methamphetamine (10 M) on Sig-1R levels in B-104 cells. B, effects of different concentrations of cocaine or methamphetamine on Sig-1R levels in B-104 cells. Cells were treated with psychostimulants for 3 days. C, effects of Sig-1R agonist (⫹) pentazocine [(⫹) PTZ] or antagonist NE-100 (NE) on the Sig-1R level in B-104 cells. (⫹) Pentazocine at 300 nM, NE-100 at 1 M. No significant differences were seen between any treated groups versus controls (one-way ANOVA). Downloaded from jpet.aspetjournals.org at ASPET Journals on August 9, 2017 we confirmed the lack of tyrosine hydroxylase, DA receptors, and DA transporter in B-104 cells (Fig. 1C). Because the detection of DA receptors using Western blotting is not always consistent and reliable using the commercial antibodies, we decided to confirm the absence of DA receptors in B-104 cells by employing functional assays detecting the formation of cAMP. We found that neither DA receptor agonists nor DA antagonists could alter cAMP levels in B-104 cells, whereas forskolin positively increased cAMP (base, ⬍1 pM; forskolin at 1 M for 10 min, 62.4 pM; dihydrexidine at 1 M for 10 min, ⬍1 pM; SCH23390 at 1 M for 10 min, ⬍1 pM; quinpirole at 1 M for 10 min, ⬍1 pM; sulpiride at 1 M for 10 min, ⬍1 pM; n ⫽ 3 for each determination). Effect of Cocaine or Methamphetamine on Sig-1R Expression. We next treated B-104 cells with cocaine or methamphetamine. B-104 cells were seeded in 1% FCS-containing DMEM and treated for 1 to 3 days with 300 nM to 10 M methamphetamine or cocaine. Sig-1R expression was unaltered under these conditions (Fig. 2, A and B). We also treated cells with the selective Sig-1R agonist (⫹)-pentazocine (300 nM) in the presence or absence of selective Sig-1R antagonist NE-100 (1 M). The agonist or the antagonist or the combination of them could not alter Sig-1R levels over a course of 3 days (Fig. 2C). None of the tendencies, if any, reached statistical significance. cAMP Down-Regulates Sig-1Rs in B-104 Cells through the Activation of PKA. In our previous study (Stefanski et al., 2004), we used a single dose of 0.5 mM cAMP and found that dB-cAMP caused an increase of Sig1Rs in NG-108 cells. In the same manner, we treated B-104 cells in the present study with 0.5 mM dB-cAMP for 1 to 3 days, with an anticipation to see an increase of Sig-1Rs. Unexpectedly, 0.5 mM dB-cAMP caused a down-regulation of Sig-1Rs in B-104 cells with an apparent peak down-regulation on day 1 (Fig. 3A). 205 206 Cormaci et al. dB-cAMP failed to affect the level of Sig-1Rs (Fig. 3F). These data indicate that the activation of the cAMP/PKA pathway causes the down-regulation of Sig-1Rs in B-104 cells. ERK Signaling up-Regulates Sig-1Rs. Inasmuch as the other main pathway regulated by cAMP in the living system is the ERK pathway, we examined the possibility that the increase of Sig-1Rs caused by 2 mM cAMP might have been through the activation of the cAMP/ERK pathway. The possibility was not far-fetched because we have found that the nerve growth factor could activate ERK and concomitantly caused the up-regulation of Sig-1Rs in PC-12 cells (Takebayashi et al., 2004). Thus, we examined whether cAMP might activate ERK in B-104 cells, specifically in a PKA-independent manner. In- deed, dB-cAMP at either 0.5 or 2 mM tended (in four determinations) to cause the activation of ERK in B104 cells in a time-dependent fashion (Fig. 4A, two left figures). dB-cAMP at 0.5 mM caused a transient ERK activation that diminished to the control level at 90 min (Fig. 4A, left). However, 2 mM dB-cAMP promoted a long-lasting ERK phosphorylation that lasted at least 90 min (Fig. 4A, middle). To confirm the potential differences, specifically at the 90-min time point, we decided to replicate the experiment at 90 min in eight determinations. Data indeed show that 2 but not 0.5 mM dB-cAMP significantly caused an increase of ERK phosphorylation at the 90-min time point (Fig. 4A, bar graph). To demonstrate that the ERK activation caused by cAMP is independent of the PKA, we used the PKA inhibitor H-89 Downloaded from jpet.aspetjournals.org at ASPET Journals on August 9, 2017 Fig. 3. Involvement of PKA in cAMP-induced Sig-1R down-regulation. A, effects of chronic treatment (1–3 days) of B-104 cells with 0.5 mM dB-cAMP on the expression of Sig-1R expression. B, dose-response curves of cAMP on the expression of Sig-1Rs in NG-108 cells (upper curve) and in B-104 cells (lower curve). C, effects of a specific PKA activator Sp-cBIMPS on the expression of Sig-1Rs. B-104 cells were treated with Sp-cBIMPS for 24 h. D, dose-dependent effect of a PKA inhibitor H-89 (200 nM), per se, on the expression of Sig-1Rs. E, time course of the effect of H-89 per se on the expression of Sig-1Rs. F, effect of H-89 on cAMP-induced Sig-1R down-regulation. B-104 cells were treated with dB-cAMP (0.5 mM) and/or H-89 (200 nM) for 24 h. ⴱ, p ⬍ 0.05; ⴱⴱ, p ⬍ 0.01; ⴱⴱⴱ, p ⬍ 0.0001 compared with controls by one-way ANOVA followed by the Tukey test. Data represents mean ⫾ S.E.M. of five to nine independent determinations. Sig-1R levels in A to E were measured by Western blotting as specified in Fig. 1. ERK Up-Regulates -1 Receptors 207 and tested if it may block the ERK activation caused by cAMP. Figure 4B shows that the ERK activation, caused either by 0.5 mM dB-cAMP at the 30-min time point (Fig. 4B, middle) or by 2 mM dB-cAMP at the 90-min time point (Fig. 4B, bottom), was not blocked by the PKA inhibitor H-89. Results are consistent with those found by Iacovelli et al. (2001). We also used PD98095, a well known inhibitor of the ERK kinase MEK, and tested if the inhibition of ERK activation might cause a down-regulation of Sig-1Rs. Indeed, PD98059 dose-dependently suppressed the ERK phosphorylation (Fig. 4C, upper) and concomitantly caused a down-regulation of Sig-1Rs (Fig. 4C, lower). Because 2 mM dB-cAMP caused a long-lasting activation of ERK, at least for 90 min (Fig. 4A, middle), the possibility exists that the long-lasting phase of ERK activation could be Downloaded from jpet.aspetjournals.org at ASPET Journals on August 9, 2017 Fig. 4. ERK signaling pathway and the up-regulation of Sig-1Rs by cAMP. A, time-dependent activation of ERK by 0.5 (left) and 2 (right) mM dB-cAMP. Phosphorylated and total ERK were measured by Western blotting using specific respective antibodies. “ERK activation” was calculated by dividing intensities of phosphorylated ERK by those from total ERK. Note that a sustained activation of ERK at 90 min is seen only in 2 mM cAMP-treated B-104 cells. Data represent means ⫾ S.E.M. of four independent determinations. A, bar graph, data from a repeat of eight independent determinations specifically on the 90-min time point. B, effects of H-89 on the cAMP-induced ERK activation caused by 0.5 (upper bar graph; at 30 min) or 2 (lower bar graph; at 90 min) mM cAMP. Data represent means ⫾ S.E.M. of six independent determinations. C, dose-dependent down-regulation of Sig-1R expression by PD98059. Data represent mean ⫾ S.E.M. of four to six independent determinations. D, effects of PD98059, added to cells at different time points, on the up-regulation of Sig-1R induced by 2.0 mM dB-cAMP. The addition of PD98059 90 min after the addition of 2 mM dB-cAMP (column 5) caused an inhibition of Sig-1R expression equivalent to that obtained from the coadministration of cAMP and PD98059 at time 0 (column 4). cAMP, db-cAMP; PD, PD98059 at 50 M. Sig-1R protein levels were assessed by Western blotting 24 h after the application of cAMP. Data represent mean ⫾ S.E.M. of six independent determinations. Sig-1Rs were measured as indicated in Fig. 1. For all panels: ⴱ, p ⬍ 0.05; and ⴱⴱ, p ⬍ 0.01, compared with controls by one-way ANOVA followed by Tukey test. For D: ⴱ, p ⬍ 0.05 compared with control; ##, p ⬍ 0.01 compared with 2 mM cAMP. 208 Cormaci et al. mainly responsible for the up-regulation of Sig-1Rs caused by 2 mM cAMP in B-104 cells. An approach to test this possibility, although not a perfect one, was to examine whether PD98059, after the establishment of the long-lasting ERK activation (i.e., 90 min after the cAMP treatment), can block the Sig-1R up-regulation. We decided to examine this possibility therefore by first allowing 2 mM cAMP to activate ERK for 90 min and then adding PD98059 to the system. The Sig-1R level was examined 24 h after the addition of cAMP. PD98059 by itself, again like that seen in Fig. 4C, attenuated the Sig-1R level (Fig. 4D, third bar from left). When coadministrated with cAMP at time 0, PD98059 blocked the Sig-1R expression (Fig. 4D, fourth bar from left). When cAMP was allowed to activate ERK for 90 min and then PD98059 was added to the system, PD98059 blocked the Sig-1R expression (bar on the right). The understanding of the basic function of Sig-lRs largely derives from testing Sig-1R ligands in physiological/pathological systems or behavioral models. Sig-1Rs were shown to be up-regulated in the brain of rats that were sensitized to methamphetamine (Takahashi et al., 2000), self-administering methamphetamine (Stefanski et al., 2004), or conditioned to cocaine-induced place preference (Romieu et al., 2004). In addition, the overexpression of Sig-1Rs in cultured cells caused morphological and functional changes of biological membranes (Hayashi and Su, 2005). However, information is very limited so far on the potential signal transduction pathways that may lead to the up- or down-regulation of Sig-1Rs. The present study, therefore, constitutes the first report describing that specific kinases downstream of cAMP, i.e., PKA and ERK, can regulate Sig-1R expression in a biological system. However, other signaling pathways may also exist to regulate the expression of Sig-1Rs in the biological system. For example, cocaine has been shown to concomitantly upregulate a transcription factor, Fra-2, and Sig-1Rs in the rat brain (e.g., Liu et al., 2005). The binding of ligands to receptors, as seen in a number of hormone and neurotransmitter systems, often causes receptor internalization, receptor degradation, as well as the activation of transcriptions, which consequently lead to the alteration of receptor expression. The intracellular distribution of Sig-1Rs has been defined recently. Sig-1Rs localize at lipid-enriched globular structures associated with endoplasmic reticulum as reported in NG-108 –15 cells (Hayashi and Su, 2003a), primary hippocampal neurons (Hayashi and Su, 2004), or oligodendrocytes (Hayashi and Su, 2004). Upon stimulation with agonists like (⫹)-pentazocine and cocaine, Sig-1Rs translocate on the ER toward perinuclear areas and the tip of neurites (Hayashi and Su, 2001, 2003b). However, cocaine, methamphetamine, or even a selective Sig-1R ligand (⫹)pentazocine did not change the Sig-1R expression as reported in this study. Cocaine levels in the post-mortem brain of cocaine addicts were estimated to be between 0.1 and 4 M (Kalasinsky et al., 2000), which is close to the Ki value of cocaine for Sig-1Rs (Sharkey et al., 1988). In this study, we used the concentrations of cocaine and methamphetamine that are close to the physiological concentrations of those drugs in human brains and found that cocaine, methamphetamine, or the selective Sig-1R agonist, (⫹)pentazocine, can- Downloaded from jpet.aspetjournals.org at ASPET Journals on August 9, 2017 Discussion not change the level of Sig-1Rs. One possible explanation might be that these drugs are difficult to pass the plasma membrane. However, it is known that (⫹)pentazocine is actively up-taken in neuronal cells in a time-, energy-, and temperature-dependent manner (Yamamoto et al., 2001). Therefore, the treatment of B-104 cells with these compounds at concentrations at least 10-fold higher than their Ki values for Sig-1Rs for 1 to 3 days at 37°C should be theoretically sufficient for ligands to bind to Sig-1Rs and alter the expression of Sig-1Rs, if any. Our results indicated otherwise. Thus, direct interaction of Sig-1R ligands with Sig-1Rs per se, even though ensuing a Sig-1R translocation (Hayashi and Su, 2003b), may not lead to transcriptional activities and/or protein degradation that may change Sig-1R levels in B-104 cells. cAMP affects a number of biological processes like cellular proliferation, differentiation, embryogenesis, and neural plasticity. The main mechanism by which cAMP exerts its cellular effects involves its activation of PKA. In our test model using B-104 cells, both db-cAMP (0.5 mM) and a PKA agonist, Sp-cBIMPS, caused a down-regulation of Sig-1Rs. These results indicate that the activation of the cAMP/PKA pathway negatively modulates the Sig-1R expression. Furthermore, our finding that H-89 alone is able to increase Sig-1Rs implicates a tonic inhibition of Sig-1R expression by PKA. The repeated treatment of psychostimulants induces sensitization in rodents and humans. The behavioral alterations induced by repeated administration of abused drugs are thought to be mediated by molecular and cellular adaptations of neurons including those in the mesolimbic dopaminergic system. In dopaminergic systems, cAMP is the primary second messenger system associated with DA receptors. Alterations in the functions of the cAMP system have been hypothesized to initiate stable neural changes associating with abuse of psychostimulants (Lu et al., 2003; Schroeder et al., 2004). As such, it is worth noting that repeated administrations of amphetamine or cocaine induce a decrease of PKA in the nucleus accumbens (Crawford et al., 2004). Inasmuch as our present data indicate that a decreased PKA activity leads to Sig-1R up-regulation, we speculate that Sig1Rs may increase in the nucleus accumbens of animals in those studies. We showed previously that Sig-1Rs are up-regulated in the methamphetamine self-administering rats, specifically in the midbrain. We did not, however, examine the PKA activity in the midbrain of those rats, nor did we examine the nucleus accumbens for Sig-1R levels. In the present study, we demonstrated that dB-cAMP can stimulate ERK in B-104 cells. cAMP has been shown to activate the ERK signaling in a PKA-dependent or -independent mechanism. Previous reports also showed that ERK could be activated by cAMP in a cell-specific manner (Stork and Schmitt, 2002). In the present study, dB-cAMPinduced ERK phosphorylation was not blocked by a PKA antagonist, H-89. This result indicates that in B-104 cells, cAMP activates ERK in a PKA-independent manner. ERK has been considered a central player in neuronal plasticity. Activation of ERK by psychostimulants could not be achieved in mice lacking dopamine- and cAMP-regulated phosphoprotein DARPP-32 (Valjent et al., 2005). Furthermore, incapacitating the ERK blocks psychostimulant-in- ERK Up-Regulates -1 Receptors expression and that up-regulation of Sig-1R seen in the brain of psychostimulant self-administering or sensitized animals may involve DA and the activation of DA receptors and associated signaling pathways in a manner that need to be totally clarified. Psychostimulants like cocaine can affect other biological systems such as voltage-gated ion channels (Nasif et al., 2005). The contribution of other biological systems, in addition to those described here, to the regulation of Sig-1R expression is unknown at present. Acknowledgments We thank F. Cambi for the generous gift of B-104 cells. References Ault DT and Werling LL (2000) SH-SY5Y cells as a model for sigma receptor regulation of potassium-stimulated dopamine release. Brain Res 877:354 –360. Aydar E, Palmer CP, Klyachko VA, and Jackson MB (2002) The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron 34:399 – 410. Crawford CA, Choi FY, Kohutek JL, Yoshida ST, and McDougall SA (2004) Changes in PKA activity and Gs␣ and Golf␣ levels after amphetamine- and cocaine-induced behavioral sensitization. Synapse 51:241–248. Hayashi T and Su TP (2001) Regulating ankyrin dynamics: roles of sigma-1 receptors. Proc Natl Acad Sci USA 98:491– 496. Hayashi T and Su TP (2003a) Sigma-1 receptors (sigma(1) binding sites) form raft-like microdomains and target lipid droplets on the endoplasmic reticulum: roles in endoplasmic reticulum lipid compartmentalization and export. J Pharmacol Exp Ther 306:718 –725. Hayashi T and Su TP (2003b) Intracellular dynamics of sigma-1 receptors (sigma(1) binding sites) in NG108 –15 cells. J Pharmacol Exp Ther 306:726 –733. Hayashi T and Su TP (2004) Sigma-1 receptors at galactosylceramide-enriched lipid microdomains regulate oligodendrocyte differentiation. Proc Natl Acad Sci USA 101:14949 –14954. Hayashi T and Su TP (2005) The potential role of sigma-1 receptors in lipid transport and lipid raft reconstitution in the brain: implication for drug abuse. Life Sci 77:1612–1624. Iacovelli L, Capobianco L, Salvatore L, Sallese M, D’Ancona GM, and De Blasi (2001) Thyrotropin activates mitogen-activated protein kinase pathway in FRTL-5 by a cAMP-dependent protein kinase A-independent mechanism. Mol Pharmacol 60: 924 –933. Itzhak Y (1993) Repeated methamphetamine-treatment alters brain sigma receptors. Eur J Pharmacol 230:243–244. Kalasinsky KS, Bosy TZ, Schmunk GA, Ang L, Adams V, Gore SB, Smialek J, Furukawa Y, Guttman M, and Kish SJ (2000) Regional distribution of cocaine in postmortem brain of chronic human cocaine users. J Forensic Sci 45:1041–1048. Liu Y, Chen GD, Lerner MR, Brackett DJ, and Matsumoto RR (2005) Cocaine up-regulates Fra-2 and sigma-1 receptor gene and protein expression in brain regions involved in addiction and reward. J Pharmacol Exp Ther 314:770 –779. Lu L, Grimm JW, Shaham Y, and Hope BT (2003) Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J Neurochem 85:1604 –1613. Lu L, Hope BT, Dempsey J, Liu SY, Bossert, and Shaham Y (2005) Central amygdale ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci 8:212–219. Maurice T, Urani A, Phan VL, and Romieu P (2001) The interaction between neuroactive steroids and the sigma1 receptor function: behavioral consequences and therapeutic opportunities. Brain Res Rev 37:116 –132. Meunier J, Gue M, Recasens M, and Maurice T (2004) Attenuation by a sigma1 (sigma1) receptor agonist of the learning and memory deficits induced by a prenatal restraint stress in juvenile rats. Br J Pharmacol 142:689 –700. Miller CA and Marshall JF (2005) Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron 15:772–775. Monnet FP, Debonnel G, Junien JL, and De Montigny C (1990) N-Methyl-Daspartate-induced neuronal activation is selectively modulated by sigma receptors. Eur J Pharmacol 179:441– 445. Nasif FJ, Hu XT, and White FJ (2005) Repeated cocaine administration increases voltage-sensitive calcium currents in response to membrane depolarization in medial prefrontal cortex pyramidal neurons. J Neurosci 25:3674 –3679. Nguyen EC, McCracken KA, Liu Y, Pouw B, and Matsomoto RR (2005) Involvement of sigma receptors in the acute actions of methamphetamine: receptor binding and behavioral studies. Neuropharmacology 49:638 – 645. Nuwayhid SJ and Werling LL (2003) Sigma1 receptor agonist-mediated regulation of N-methyl-D-aspartate-stimulated [3H]dopamine release is dependent upon protein kinase C. J Pharmacol Exp Ther 304:364 –369. Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio JM, Rothman RB, Su TP, Tam SW, and Taylor DPA (1992) A proposal for the classification of sigma binding sites. Trends Pharmacol Sci 13:85– 86. Robin P, Rossignol B, and Raymond MN (1998) PKA inhibitor, H-89, affects the intracellular transit of regulated secretory proteins in rat lacrimal glands. Am J Physiol 274:C262–C271. Romieu P, Martin-Fardon R, Bowen WD, and Maurice T (2003) Sigma-1 receptorrelated neurosteroids modulate cocaine-induced reward. J Neurosci 23:3572–3576. Romieu P, Meunier J, Garcia D, Zozime N, Martin-Fardon R, Bowen WD, and Maurice T (2004) The sigma-1 receptor activation is a key step for the reactivation Downloaded from jpet.aspetjournals.org at ASPET Journals on August 9, 2017 duced sensitization and rewarding effects (Lu et al., 2005; Miller and Marshall, 2005). Thus, the ERK signaling pathway may be tightly involved in the neuronal adaptations underpinning psychostimulant-induced behavioral alternations. However, the cellular biological sequelae downstream of the ERK activation have not been totally clarified. In the present study, we show that ERK activation can cause the up-regulation of Sig-1Rs. Furthermore, in the time course experiment, we found that 0.5 mM dBcAMP transiently activates ERK (returning to basal level within 90 min), whereas 2 mM dB-cAMP causes a longlasting ERK phosphorylation (lasting over 90 min). Blockade of the long-lasting phase of ERK activation by applying PD98059 at 90 min after 2.0 mM dB-cAMP completely attenuates the up-regulation of Sig-1Rs. These results suggest that the long-lasting activation of ERK may be important for inducing the up-regulation of Sig-1Rs. However, the involvement of the transient ERK activation cannot be totally ruled out at present because the activation, although transient, might have caused a late secondary effect affecting Sig-1R expression. Repetitive or chronic use of psychostimulants thus could promote the long-lasting activation of ERK that may in turn up-regulate Sig-1Rs. Our results suggest that the homeostasis of PKA and ERK play an important role in the regulation of Sig-1R expression. Psychostimulants may affect neuroplasticity by altering the homeostasis between PKA and ERK. We do not know at present exactly how 0.5 and 2 mM dB-cAMP differentially affect the PKA and ERK systems. It is possible that in NG-108 cells, the ERK pathway up-regulating Sig-1R may prevail over the PKA pathway. ERK causing the up-regulation of Sig-1Rs may represent an important cellular biological event in the addictive processes. As mentioned before, Sig-1R up-regulation causes a reconstitution of lipid rafts by increasing cholesterol into the raft region (Takebayashi et al., 2004), increases cellular differentiation (Takebayashi et al., 2002), and potentiates brain-derived neurotrophic factor signaling for glutamate release (Yagasaki et al., 2006). Furthermore, Sig-1R antagonists block behavioral sensitization to methamphetamine (Takahashi et al., 2000) and block the relapse to cocaineinduced conditioned place preference (Romieu et al., 2003). Thus, ERK activation up-regulating Sig-1Rs may represent an important signaling sequela underlying the psychostimulant-induced addictive behavior. In summary, we have successfully identified cAMP-mediated signal pathways that control the Sig-1R expression. cAMP is apparently bimodal in regulating the expression of Sig-1Rs in B-104 cells, namely, via a PKA activation-dependent down-regulation and via an ERK activation-dependent up-regulation. It is interesting to note that repetitive administration of cocaine or methamphetamine decreases PKA activity (Crawford et al., 2004), whereas it increases ERK activity in animals (Lu et al., 2005; Miller and Marshall, 2005). According to the results of our present study, the decreased PKA activity coupled with an increase in ERK activity will presumably lead to an increase of Sig-1Rs in the brain. Increases of Sig-1Rs in the brain may in turn restructure neurons toward a long-lasting addictive state, perhaps via Sig1R’s action on trophic factors or via Sig-1R’s ability to reconstitute lipid rafts on the plasma membrane. Our results also suggest that ligand binding per se may not affect Sig-1R 209 210 Cormaci et al. mal growth factor signaling towards neuritogenesis in PC12 cells: potential relation to lipid raft reconstitution. Synapse 53:90 –103. Ujike H, Kuroda S, and Otsuki S (1996) Sigma receptor antagonists block the development of sensitization to cocaine. Eur J Pharmacol 296:123–128. Valjent E, Corbille AG, Bertran-Gonzalez J, Herve D, and Girault JA (2006a) Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci USA 103: 2932–2937. Valjent E, Corvol JC, Trzaskos JM, Girault JA, and Herve D (2006b) Role of the ERK pathway in psychostimulant-induced locomotor sensitization. BMC Neurosci 7:20. Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, et al. (2005) Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA 102:491– 496. Yagasaki Y, Numakawa T, Kumamaru E, Hayashi T, Su T-P, and Kunugi H (2006) Chronic antidepressants potentiate via sigma-1 receptors the brain-derived neurotrophic factor-induced signaling for glutamate release. J Biol Chem 281:12941– 12949. Yamamoto H, Karasawa J, Sagi N, Takahashi S, Horikomi K, Okuyama S, Nukada T, Sora I, and Yamamoto T (2001) Multiple pathways of sigma(1) receptor ligand uptakes into primary cultured neuronal cells. Eur J Pharmacol 425:1–9. Address correspondence to: Dr. Tsung-Ping Su, Cellular Pathobiology Unit, Triad Building (Room 3304), 333 Cassel Drive, Baltimore, MD 21224. E-mail: [email protected] Downloaded from jpet.aspetjournals.org at ASPET Journals on August 9, 2017 of cocaine conditioned place preference by drug priming. Psychopharmacology 175:154 –162. Schroeder JA, Hummel M, and Unterwald EM (2004) Repeated intracerebroventricular forskolin administration enhances behavioral sensitization to cocaine. Behav Brain Res 153:255–260. Severson JA, de Vellis JS, and Finch CE (1983) [3H]spiperone binding sites in rat primary glial cultures, C6 glioma and B104 neuroblastoma. J Neurosci Res 9:21– 26. Sharkey J, Glen KA, Wolfe S, and Kuhar MJ (1988) Cocaine binding at sigma receptors. Eur J Pharmacol 149:171–174. Snyder SH and Largent BL (1989) Receptor mechanisms in antipsychotic drug action: focus on sigma receptors. J Neuropsychiatry Clin Neurosci 1:7–15. Stefanski R, Justinova Z, Hayashi T, Takebayashi M, Goldberg SR, and Su TP (2004) Sigma1 receptor upregulation after chronic methamphetamine self-administration in rats: a study with yoked controls. Psychopharmacology 175:68 –75. Stork PJ and Schmitt JM (2002) Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol 12:258 –266. Su TP, London ED, and Jaffe JH (1988) Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science (Wash DC) 240:219 –221. Takahashi S, Miwa T, and Horikomi K (2000) Involvement of sigma 1 receptors in methamphetamine-induced behavioral sensitization in rats. Neurosci Lett 289:21– 24. Takebayashi M, Hayashi T, and Su TP (2002) Nerve growth factor-induced neurite sprouting in PC12 cells involves sigma-1 receptors: implications for antidepressants. J Pharmacol Exp Ther 303:1227–1237. Takebayashi M, Hayashi T, and Su T-P (2004) Sigma-1 receptors potentiate epider-









![Subject: Camp Vendini conference Hi [First name], I`d like to request](http://s1.studyres.com/store/data/020114553_1-b2260bcb92a35a8631722149325729b5-150x150.png)