* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download OXIDATIVE PHOSPHORYLATION
Nicotinamide adenine dinucleotide wikipedia , lookup
Mitochondrial replacement therapy wikipedia , lookup
Metalloprotein wikipedia , lookup
Photosynthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Mitochondrion wikipedia , lookup
Phosphorylation wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Microbial metabolism wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Citric acid cycle wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
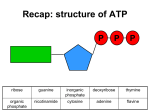
OXIDATIVE PHOSPHORYLATION 9/25/07 ATP _ Universal Carrier of Free Energy Provides Energy for: _ Mechanical _ Chemical Work _ Ionic Role of ATP in Metabolism Know this structure It is the most important molecule in biochemistry. The Concept Energy rich molecules donate electrons to specific coenzymes to form energy-rich reduced coenzymes 1 pair of electrons is donated per each reduced coenzyme H2 = H + H H = 1 electron + 1 proton :H¯ = 2 electrons + 1 proton (Hydride ion) + + H = 1 proton These electrons are donated to the electron transport chain to form ATP Electrochemical gradient Oxidized Reduced Carriers and transport systems are used to move ions and molecules across this membrane Glycolysis Cytoplasm • Membrane convoluted or folded = ↑ Surface Area (Cristae) Electron Transport Contains the ATP Synthetase complex • Inner mitochondrial membrane • Is the final common pathway by which electrons from food molecules are used to make ATP and molecular oxygen acts as the final acceptor of the electrons ATP Synthetase complex 50% protein NADH Dehydrogenase Final acceptor of e-s is molecular oxygen Citric acid cycle 2 e¯ Citric acid cycle 2 e¯ Cytochrome oxidase (Iron + copper) Complex V contains ATP Sythase • Series of Oxidation/Reduction reactions Electron transport chain ▬ 3 components • Flavoprotein ▬ NADH Dehydrogenase • CoQ (Quinone) ▬ Ubiquinone • Cytochromes ▬ Heme group ▬ Iron Ferric (Fe3+) ▬ Ferrous (Fe2+) ▬ Cyanide Each one of these inhibitors will completely stop electron transport and thus all ATP production Three Main Tenets of the Mitchell Theory ATP Synthase FAD+ FADH2 This dissipates gradient Blocked by atractyoside (Plant toxin) ATP Oligomycin blocks Lower pH pH gradient More protons Electrical gradient ADP ATP Cytoplasm 1 NADH = 3 ATP 1 FADH2 = 2 ATP Chemiosmotic Hypothesis of Electron Transport coupled to ADP Phosphorylation ▬ “Mitchell Hypothesis” Features: • Protons transported from the matrix to the inner mitochondrial space results in an electric gradient and a pH gradient • As the protons flow through the membrane channel back into the matrix they drive ATP synthesis Occurs with energy utilized by ATP synthase This proton transport couples electron transport to oxidative phosphorylation Uncoupling of Oxidative Phosphorylation O2 Electron Transport ADP ADP Electron transport coupled to phosphorylation of ADP ATP ADP H2O ATP ATP ATP Introduced in 1932 as weight reduction drug = Fatal hyperthermia + Dinitrophenol (DNP) breaks down proton gradient High doses of aspirin ▬ results in fever O2 Electron transport continues Electron Transport No ADP phosphorylation Energy dissipated as heat H2O HEAT Brown adipose tissue creates heat by thermogenesis Thermogenin = uncoupling protein ▬ UCP1 The energy is given off as heat Mechanism is to ↑ FA oxidation which uncouples oxidation phosphorylation Breaks down proton gradient Inherited Diseases of Oxidative Phosphorylation LIBER’S HEREDITARY OPTIC NEUROPATHY Bilateral loss of central vision occurs because of Neuroretinal degeneration Mutation in mitochondrial DNA Mitochondrial DNA is maternally inherited because all mitochondria come from the mother None come from the sperm because none from sperm enter the egg during fertilization Some mitochondrial myopathies caused by mutations in mtDNA 13 of the ~100 proteins in the mitochondrion are coded for by mtDNA Has mutation rate > 10x that of nuclear DNA Glycerophosphate shuttle 2 ATP for each NADH transported into mitochondrion from the cytoplasm Malate-Aspartate shuttle 3 ATP for each NADH 1 2 3 6 4 7 5 2 Shuttle systems to bring cytosolic NADH into mitochondria for oxidative phosphorylation 1) Glycerophosphate shuttle = 36 ATP 8 2) Malate-aspartate shuttle = 38 ATP Count ATPs: Anerobic glycolysis = 2 Glycolysis + CAC + oxidative phosphorylation = 38 NADH FADH2 ATP 1 Glycolysis 2 Glycolysis (G-3-P 1,3,BisP) 2 6 3 Pyruvate Acetyl CoA 2 6 4, 5, 6 CAC 6 18 7 CAC-FADH2 8 CAC – substrate level ATP Total 2 2 4 2 38 Revolves at 100 Hz (revolutions/s) This is sufficient to produce a turnover of The weight of our body of ATP each day! ATP synthase: • 2 Domains F1 F0 • Rotates in 120° stages • 100 Hz one complete revolution = 3 ATP • Need 10 H+ . . . 1 ATP = 3.3 H+