* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Respiration
Nicotinamide adenine dinucleotide wikipedia , lookup
Metalloprotein wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Photosynthesis wikipedia , lookup
Phosphorylation wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Mitochondrion wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Biochemistry wikipedia , lookup
Microbial metabolism wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Electron transport chain wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
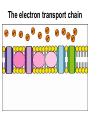
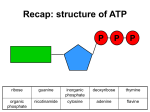
Recap: structure of ATP P P P ribose guanine inorganic phosphate deoxyribose thymine organic phosphate nicotinamide cytosine adenine flavine Recap: where in the cell? 1. Glycolysis 1. Phosphorylation 2. Oxidation 2. Link reaction 3. Krebs cycle 4. Oxidative phosphorylation Recap: glycolysis Recap: link reaction Recap: Krebs cycle Enzymes • Glycolysis: – Phosphofructokinase • Krebs cycle: – Decarboxylases – Dehydrogenases Regulatory enzymes glucose ↑ ATP ↑ citrate phosphofructokinase enzyme pyruvate Krebs cycle ↑ ADP ↓ ATP ↓ citrate How much ATP has been produced? • Glycolysis: • Link reaction: • Krebs cycle: Is this enough??? The electron transport chain The electron transport chain 1. Hydrogen atoms released from NADH and FADH as they are oxidised 2. Hydrogen atoms split into protons and electrons 3. Electrons move along the electron transport chain, losing energy at each carrier 4. Energy is used to pump protons into intermembrane space forming an electrochemical gradient 5. Protons move down electrochemical gradient back to matrix via ATP synthase 6. Movement of protons drives synthesis of ATP from ADP and inorganic phosphate 7. Protons, electrons and oxygen combine to form water, the final electron acceptor Evidence for chemiosmosis 1. pH of intermembrane space is lower than pH of matrix – Proton gradient exists between intermembrane space and matrix 2. Artificial vesicles created with proton pump proteins resulted in ↓ pH in vesicle – Proton gradient can be used to synthesise ATP 3. Mitochondria in pH8 solution produced no ATP – Proton gradient can be used by mitochondria to make ATP 4. Uncouplers destroyed proton gradient in mitochondria – Proton gradient is required by mitochondria to make ATP How much ATP? • Oxidative phosphorylation makes ATP using energy from NADH and FADH • 1 NADH → 2.5 ATP • 1 FADH → 1.5 ATP More cutbacks: In 1997 1 NADH → 3 ATP and 1 FADH → 2 ATP! How much ATP? Stage of respiration Molecules produced Number of ATP molecules Glycolysis Link reaction (x2) Krebs cycle (x2) Total ATP = Anaerobic respiration glucose pyruvate carbon dioxide + ethanal ethanol lactic acid Aerobic and anaerobic respiration Aerobic Anaerobic • Where? • Where? • Is oxidation complete? • Is oxidation complete? • What are the waste products? • What are the waste products? • How much energy? • How much energy?