* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Glycolysis - Fairfield Public Schools
Signal transduction wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Metalloprotein wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Mitochondrion wikipedia , lookup
Phosphorylation wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Photosynthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Microbial metabolism wikipedia , lookup
Biochemistry wikipedia , lookup
Electron transport chain wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
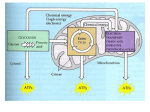
Cellular Respiration Cellular respiration Aerobic respiration Requires molecular oxygen Includes redox reactions Anaerobic Anaerobic respiration Fermentation Do not require oxygen All exergonic Energy flows into an ecosystem as sunlight and leaves as heat Photosynthesis generates O2 and organic molecules, which are used in cellular respiration Cells use chemical energy stored in organic molecules to regenerate ATP, which powers work Catabolic pathways yield energy by oxidizing organic fuels The breakdown of organic molecules is exergonic Fermentation is a partial degradation of sugars that occurs without O2 Aerobic respiration consumes organic molecules and O2 and yields ATP Anaerobic respiration is similar to aerobic respiration but consumes compounds other than O2 Catabolic Pathway Complex organic molecules Catabolic Pathway Simpler waste products with less E Some E used to do work and dissipated as heat Cellular respiration includes both aerobic and anaerobic respiration but is often used to refer to aerobic respiration Although carbohydrates, fats, and proteins are all consumed as fuel, it is helpful to trace cellular respiration with the sugar glucose C6H12O6 + 6 O2 6 CO2 + 6 H2O + Energy (ATP + heat) Redox Reactions: Oxidation and Reduction The transfer of electrons during chemical reactions releases energy stored in organic molecules This released energy is ultimately used to synthesize ATP The Principle of Redox Chemical reactions that transfer electrons between reactants are called oxidationreduction reactions, or redox reactions • In oxidation, a substance loses electrons, or is oxidized In reduction, a substance gains electrons, or is reduced (the amount of positive charge is reduced) During cellular respiration, the fuel (such as glucose) is oxidized, and O2 is reduced Cellular respiration includes both aerobic and anaerobic respiration but is often used to refer to aerobic respiration Although carbohydrates, fats, and proteins are all consumed as fuel, it is helpful to trace cellular respiration with the sugar glucose C6H12O6 + 6 O2 6 CO2 + 6 H2O + Energy (ATP + heat) Stepwise Energy Harvest via NAD+ and the Electron Transport Chain In cellular respiration, glucose and other organic molecules are broken down in a series of steps Electrons from organic compounds are usually first transferred to NAD+, a coenzyme As an electron acceptor, NAD+ functions as an oxidizing agent during cellular respiration Each NADH (the reduced form of NAD+) represents stored energy that is tapped to synthesize ATP Four stages of aerobic respiration Takes place in the cytosol Glycolysis Takes place in the mitochondrion Formation of acetyl CoA Citric acid cycle Electron transport chain/chemiosmosis Four stages of aerobic respiration Summary of aerobic respiration Glycolysis “Sugar splitting” Does not require oxygen Divided into two major phases Energy investment phase Energy capture phase Each glucose molecule produces net yield of two NADH molecules and two ATP molecules Substrate-level phosphorylation Glycolysis INPUTS Glucose 2 ATP 4 ADP 2 P 2 NAD+ Outputs 2 Pyruvate 2 ADP 4 ATP 2 NADH + H+ Glycolysis Glycolysis Occurs in the cytoplasm. Glycolysis is anaerobic - requires no O2. Energy in the form of 2 ATP is gained. Energy in the form of 2NADH + H+ is gained. Each NADH + H+ yields 3 ATP at the ETC for a total of 6 ATP. 2 Pyruvate are gained which are modified where further energy can be extracted. Glycolysis In the presence of O2, pyruvate enters the mitochondrion (in eukaryotic cells) where the oxidation of glucose is completed The Mitochondrion Outer membrane contains large-pore channel proteins. Highly folded selective inner membrane contains the mitochondrial matrix. Many enzymes of the CAC are dissolved in the matrix with the rest being attached to the inner face of the inner membrane. This membrane also contains ET molecules. Mito. Cont. Inner membrane helps to create an electrochemical gradient by pushing H+ ions outside the membrane. Inner membrane contains ATP synthetase enzymes. Aerobic bacteria membrane molecules occur in the plasma membrane Conversion of Pyruvate to Acetyl Co-A Pyruvate crosses the inner mitochondrial membrane into the matrix. Pyruvate is oxidized and decarboxylated. NAD picks up a hydrogen atom and electron and an H+ follows in solution. CO2 leaves the remaining 2-C acetyl binds to Co-A Oxidation of Pyruvate to Acetyl CoA Before the citric acid cycle can begin, pyruvate must be converted to acetyl Coenzyme A (acetyl CoA), which links glycolysis to the citric acid cycle This step is carried out by a multienzyme complex that catalyses three reactions Conversion of Pyruvate to Acetyl Co-A Inputs 2 Pyruvate 2 NAD 2 Co-A Outputs 2 CO2 2 NADH + H+ (for a total gain of 6 ATP at the ETC) 2 acetyl Co-A Formation of acetyl CoA Citric Acid Cycle The molecules are structurally rearranged to strip off H+ ions to be used in oxidative phosphorylation. A total of 2 ATP molecules are formed by substrate level phosphorylation. The cycle oxidizes pyruvate to 6 NADH, and 2 FADH2 The remaining carbon and oxygen are released as CO2 (4 CO2 total). Citric Acid Cycle 2 2 2 2 6 2 Inputs Oxaloacetate Acetyl Co-A ADP P NAD+ FAD 2 2 4 2 6 2 Outputs Oxaloacetate Co-A CO2 ATP NADH + H+ 18 ATP FADH2 4 ATP Citric acid cycle Oxidative PhosphorylationChemiosmosis Electron transfer in the electron transport chain causes proteins to pump H+ from the mitochondrial matrix to the intermembrane space H+ then moves back across the membrane, passing through the proton, ATP synthase ATP synthase uses the exergonic flow of H+ to drive phosphorylation of ATP This is an example of chemiosmosis, the use of energy in a H+ gradient to drive cellular work Accumulation of protons within the inter-membrane space Electron transport and chemiosmosis The Electron Transport System e- arriving at the end have given up most of their energy. O2 takes these e- plus some H+ forming new water molecules. 4e- + 4H+ + O2 2H2O The H+ Gradient and Phosphorylation -?? Electrochemical potential energy 220 millivolts difference - electrical. H+ pH difference - chemical. Proton-motive force - hydrogen ions are protons coupled with the force of them moving back across the membrane. Chemiosmotic ATP Synthesis Coupling between the flow of H+ down its gradient and the phosphorylation of ADP. ATP synthetase can make ATP without the flow of H+. It can not release the ATP until H+ ions flow flow through the chanel toward the matrix, down their electrochemical gradient. H+ gradient powers ATP synthesis indirectly by freeing the enzymes active site. Summary of Oxidation Energy is stockpiled in the form of an H+ electrochemical gradient across the inner mitochondrial membrane, via a series of oxidation-reduction reactions. The electron transport system accepts hydrogens from NADH and FADH2. The system passes the hydrogens’ electrons through a series of redox reaction and uses the energy released to pump H+ through the membrane against its electrochemical gradient. At the end of the system, the e- are accepted by O2, which also picks up H+ to form water. Summary of Phosphorylation The energy of the H+ gradient is used in the phosphorylation of ADP to ATP. This happens as H+ re-crosses the membrane via channel proteins connected to ATP synthetase enzymes. Maximum ATP Yield Process Number of Reduced H Carriers Maximum Number of ATP per H Pair Number of ATP by Oxidative Net ATP by substrate level Glycolysis 2 NADH + 3 ATP/2H 6 ATP 2 ATP Pyruvate to AcetylCoA Citric Acid 6 NADH + + H Cycle 6 ATP H+ 2 NADH + 3 ATP/2H H+ Grand 2 FADH2 Total 3 ATP/2H 2 ATP/2H Phosphorylation Phosphorylation 18 ATP 4 ATP 2 ATP 34 ATP 4 ATP Energy yield from complete oxidation of glucose by aerobic respiration Many organisms depend on nutrients other than glucose Products of protein and lipid catabolism enter same metabolic pathways as glucose Amino acids are deaminated Energy from carbohydrates, proteins, and fats Fermentation Electron transport molecules get stuck in the reduced form, holding electrons. System stops working. H+ gradient is used up. Fermentation uses organic molecules as final electron acceptors. Pyruvate can serve as an alternative electron acceptor to free up NADH Aerobic respiration versus fermentation Aerobic respiration Electrons transferred from fuel molecules to electron transport chain Final electron acceptor is inorganic substance Fermentation Anaerobic process that does not use electron transport chain Comparison of aerobic respiration, anaerobic respiration, and fermentation Fermentation Glycolysis: energy investment phase Glycolysis: energy capture phase Detail of citric acid cycle