* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download METABOLISM IN HEALTH AND DISEASES I Lecture 2 Pentose
Mitogen-activated protein kinase wikipedia , lookup
Metalloprotein wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Isotopic labeling wikipedia , lookup
Microbial metabolism wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Photosynthesis wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Paracrine signalling wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Biochemical cascade wikipedia , lookup
Citric acid cycle wikipedia , lookup
Biochemistry wikipedia , lookup
Amino acid synthesis wikipedia , lookup
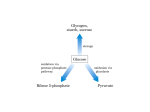
BCHEM 254 METABOLISM IN HEALTH AND DISEASES II Lecture 2: Pentose Phosphate Pathway Lecturer: Christopher Larbie, PhD • In most animal tissues, glucose is catabolized via the glycolytic pathway into two molecules of pyruvate. • Pyruvate is then oxidized via the citric acid cycle to generate ATP. • There is another metabolic fate for glucose used to generate NADPH and specialized products needed by the cell. • This pathway is called the pentose phosphate pathway. • Some text books call it the hexose monophosphate shunt, still others call it the phosphogluconate pathway. • The pentose phosphate pathway produces NADPH which is the universal reductant in anabolic pathways • In mammals the tissues requiring large amounts of NADPH produced by this pathway are the tissues that synthesize fatty acids and steroids such as the mammary glands, adipose tissue, adrenal cortex and the liver. • Tissues less active in fatty acid synthesis such as skeletal muscle are virtually lacking the pentose phosphate pathway. • The second function of the pentose phosphate pathway is to generate pentoses, particularly ribose which is necessary for the synthesis of nucleic acids. The Pentose Phosphate Pathway (PPP • It is convenient to think of the pentose phosphate pathway as operating in two phases. • The first phase is the oxidative phase. Two of the first three reactions of the first phase generate NADPH. The second phase is the nonoxidative phase. • In the first step glucose-6-phosphate is oxidized into ribulose-5-phosphate and CO2. • During the oxidation of glucose-6-phosphate, NADP+ is reduced into NADPH. • The second step of the pathway coverts the ribulose 5- phosphate into other pentose-5- phosphates including ribose-5-phosphate used to synthesize nucleic acids. • The third step includes a series of reactions that convert three of the pentose-5-phosphates into two molecules of hexoses and one triose. • In the fourth step, some of these sugars are converted into glucose-6-phosphate so the cycle can be repeated. • The direction of the pathway varies to meet different metabolic conditions. Oxidative Phase • The oxidative phase of the pentose phosphate pathway is composed of three steps; 1. Glucose-6-Phosphate dehydrogenase (G6PD) • • reaction: The PPP begins with the oxidation of glucose-6phosphate to produce a cyclic ester (the lactone of phosphogluconic acid) and NADPH. G6PD is highly specific for NADP+. This reaction is irreversible and highly regulated by NADPH and fatty acid esters of coenzyme A (which are intermediates of fatty acid biosynthesis). • Inhibition by NADPH is dependent on the cytosolic NADP+/NADPH ratio (in the liver it’s about 0.015). 2. Gluconolactonase reaction: • The gluconolactone produced in step 1 is hydrolytically unstable and readily undergoes a spontaneous ringopening hydrolysis, although the enzyme accelerates the reaction • The product is 6-phospho-D-gluconate, a sugar acid which is further oxidized in the next step 3. 6-phosphogluconate dehydrogenase reaction • This enzyme catalyses the oxidative decarboxylation of 6phosphogluconate to yield D-ribolus-5-phosphate and NADPH. • This reaction occurs in two distinct steps: • The initial NADP+-dependent dehydrogenation yields a β- keto acid, 3-ketp-6-phosphogluconate, which is very susceptible to decarboxylation (the second step). • The product of the reaction, riboluso-5-phosphate is the substrate for the non-oxidative reactions. Nonoxidative Phase • The nonoxidative phase of the pentose phosphate pathway is composed of 5 steps but only 4 types of reactions. • This portion of the pathway begins with an isomerization and epimerization, and it leads to the formation of either Dribose-5-phosphate or D-xylulose5-phosphate. • These intermediates can then be converted into glycolytic intermediates or directed to biosynthetic processes 4. Phosphopentose Isomerase Reaction • This enzyme converts ribulose-5-phosphate and ribose-5phosphate via an edediol intermediate. • The ribose-5-phosphate produced in this reaction is utilized in the biosynthesis of coenzyems (including NADH, NADPH, FAD and B12), nucleatides and nucleic acids (DNA and RNA). The net reaction to this point is Glucose-6-phosphate + 2NADP+ → ribose-5-phosphate + CO2 + 2NADPH + 2H+ 5. Phosphopentose Epimerase Reaction • This enzyme converts riboluse-5-phosphate to another ketose, xylulose-5-P proceeded by an enediol intermediate at C-3. • In the reaction, an acidic proton located α- to a carbonyl carbon is removed to generate the enediolate, but the proton is added back to the same carbon from the opposite side. • From the beginning to this point, 2 NADPH has been generated or every molecule of glucose-6-P converted to pentose-5-P • The next three steps, there will be rearrangement to generate three-, four-, six- and seven-carbon units, which can be utilized in various metabolic processes • This is important because, very often the cellular need for NADPH is considerably greater than the need for ribose5-P • The next three steps thus return some of the 5-C units to glyceraldehyde-3-P and fructose-6-P, which can enter the glycolytic pathway • The advantage of this is that the cells have met its need for NADPH and ribose-5-phosphate in a single pathway, yet at the same time it can return excess carbon metabolites to glycolysis 6. Transketolase Reaction • This enzyme catalysis the transfer two carbon unit from xylulose-5-P to ribose-5-P yielding 3-C unit glyceraldehyde-3P and sedoheptulose-7-P • The donor molecule is a ketone and the acceptor molecule is an aldose. G-3-P enters the glycolytic pathway. • Transketolase is dependent on thiamine pyrophosphate as a cofactor. • The mechanism involves the abstraction of the acidic thiozole proton of TPP, attack by the resulting carbanion at the carbonyl carbon of the ketose phosphate substrate, expulsion of the G-3-P product, and transfer of the 2 C unit. • Transketolase can process a variety of 2-keto sugar substrates in a similar manner as in reaction 8 7. Transaldolase Reaction • This enzyme catalysis the conversion of S-7-P and G-3-P to erthrose-4-P and fructose-6-P. • This reaction is similar to the aldose reaction of glycolysis. It involves the formation of a Schiff base intermediate between the S-7-P and an active-site lysine residue. • Elimination of the E-4-P product leaves an enamine of dihydroxyacetone, which remains stable at the active site until the other substrate comes into position. • Attack of the enamine carbanion at the carbonyl of G-3-P is followed by hydrolysis of the Schiff base (imine) to yield the product fructose-6-phosphate. 8. Transketolase Reaction • This reaction is similar to Step 6. Here the transketolase catalysis the transfer of a 2C unit from X-5-P to E-4-P yielding G-3-P and fructose-6-P. • Both products enter the glycolytic pathway Net Reaction • Oxidative phase: • 3 Glucose-6-phosphate + 6 NADP+ → 2 Xylulose-5p + ribose-5-P + 3CO2 + 6NADPH + 6H+ • Rearrangements of the non-oxidative phase: • 2 Xylulose-5-P + ribose-5-P → 2 Frucose-6P + Glyceraldehyde-3-P • The sum of these two phases: • 3 Glucose-6-phosphate + 6 NADP+ →2 Frucose-6-P + Glyceraldehyde-3-P + 3 CO2 + 6 NADPH + 6 H Tailoring the Pentose Phosphate Pathway to meet specific needs of the cell 1. If the cell requires both ribose-5-P and NADPH, the first four reactions of the PPP predominate. • NADPH is produced by the oxidative reactions of the pathway, and ribose-5-P is the principal product of carbon metabolism 2. More ribose-5 phosphate needed than NADPH • This can be can be accomplished with the synthesis of ribose-5-P with NADPH by by-passing the oxidative steps. • This is achieved by the withdrawal of F-6P and G-3-P, but not glucose-6-P, from glycolysis. • The reaction of transketolase and transaldolase on F-6-P and G-3-P produces three molecules of ribose-5-P from two molecules of F-6-P and one G-3-P. • In this route, no carbon metabolites return to glycolysis. 5 G-6-P + ATP → 6 R-5-P + ADP + Pi 3. More NADPH needed than Ribose-5-phosphate • Large amounts of NADPH can be supplied in the PPP without concomitant production of ribose-5-P if ribose-5-P produced in the PPP is recycled to produce glycolytic intermediates. • This alternative involves a complex interplay between the transketolase and transaldolase reactions to convert ribulose-5-P to F-6-P by gluconeogenesis. • The net reaction is 6 G-6-P + 12 NADP+ + 6 H2O → 6 Ribulose-5-P + 6 CO2 + 12 NADPH + 12 H+ 6 Ribulose-5-P → 5 Glucose-6-P + Pi Net: Glucose-6-P + 12 NADP+ + 6 H2O → 6 CO2 + 12 NADPH + 12H+ + Pi 4. Both NADPH and ATP are needed but not ribose-5-phosphate • Under some conditions, both NADPH and ATP must be provided in the cell. • This can be accomplished in a series of reactions similar in case 3 if the F-6-P and G-3-P produced in this way proceed through glycolysis to produce ATP and pyruvate, which itself can yiled even more ATP by continuing on to the TCA cycle. • The net reaction for this alternative is 3 Glucose-6-P + 5 NAD+ + 6 NADP+ + 8 ADP + 5 Pi → 5 Pyruvate + 3 CO2 + 5 NADH + 6 NADPH + 8 ATP + 2 H2O + 8 H+ Regulation • The first step of the phosphopentose pathway is the irreversible committed step. • This reaction is catalysed by glucose-6-phosphate dehydrogenase • This step is of course allosterically regulated. The product of this reaction NADPH is a strong inhibitor • So when the cytosol concentration of NADPH is high, the enzyme’s activity is low. It is also allosterically regulated by fatty acid acyl esters of coenzyme A • The transcription of the gene for this enzyme is under hormonal regulation • Xylulose-5-P also serves as a signalling molecule. • When blood glucose levels rise, glycolysis and PPP pathways are activated in the liver and the X-5-P produced in the latter pathways stimulate protein phosphate 2A (PP2A) • The (PP2A) dephosphorylate the bifunctional enzyme phosphofructokinase-2 or fructose-2,6-bisphosphatase • Increased levelf of F-2,6-BP stimulate glycolysis and inhibit gluconeogenesis. • At the same time, PP2A dephosphorylates carbohydrate responsive element –binding protein (ChREBP), a transcription factor that activates expression of liver genes for lipid synthesis. • These effects are a powerful combination. Increased glycolysis produces substantial amounts of acetyl-CoA, the principal substrate for lipid synthesis. • The PPP produced NADPH, the source of electrons for lipid biosynthesis. • Elevated expression of the appropriate genes sets the stage for lipid biosynthesis in the liver, an important consequence of ingestion of carbohydrates Glucose-6-phosphate dehydrogenase deficiency • Glucose-6-phosphate dehydrogenase deficiency (G6PD) is an X-linked recessive hereditary disease characterized by abnormally low levels of glucose-6-phosphate dehydrogenase, especially important in red blood cell metabolism. • G6PD deficiency is the most common human enzyme defect. • Individuals with the disease may exhibit non- immune haemolytic anaemia in response to a number of causes, most commonly infection or exposure to certain medications or fava beans. • Glucose-6-phosphate dehydrogenase (G6PD) is an enzyme in the pentose phosphate pathway. • G6PD converts glucose-6-phosphate into 6-phosphogluconoδ-lactone • This is the rate-limiting enzyme of this metabolic pathway that supplies reducing energy to cells by maintaining the level of the co-enzyme nicotinamide adenine dinucleotide phosphate(NADPH) • The NADPH in turn maintains the supply of reduced glutathione in the cells that is used to mop up free radicals that cause oxidative damage. • The G6PD / NADPH pathway is the only source of reduced glutathione in red blood cells (erythrocytes) • The role of red cells as oxygen carriers puts them at substantial risk of damage from oxidizing free radicals except for the protective effect of G6PD/NADPH/glutathione • People with G6PD deficiency are therefore at risk of haemolytic anaemia in states of oxidative stress • Oxidative stress can result from infection and from chemical exposure to medication and certain foods. • Broad beans, e.g., fava beans, contain high levels of vicine, divicine, convicine and isouramil, all of which are oxidants • When all remaining reduced glutathione is consumed, enzymes and other proteins (including haemoglobin) are subsequently damaged by the oxidants, leading to electrolyte imbalance, cross-bonding and protein deposition in the red cell membranes. • Damaged red cells are phagocytosed and sequestered (taken out of circulation) in the spleen. • The haemoglobin is metabolized to bilirubin (causing jaundice at high concentrations). • The red cells rarely disintegrate in the circulation, so haemoglobin is rarely excreted directly by the kidney, but this can occur in severe cases, causing acute renal failure • Deficiency of G6PD in the alternative pathway causes the build-up of glucose and thus there is an increase of advanced glycation end-products (AGE) • The deficiency also reduces the amount of NADPH, which is required for the formation of nitric oxide (NO) • Although female carriers can have a mild form of G6PD deficiency (dependent on the degree of inactivation of the unaffected X chromosome), homozygous females have been described • In these females there is co-incidence of a rare immune disorder termed chronic granulomatous disease (CGD)