* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Immunity to infection
Neonatal infection wikipedia , lookup
Social immunity wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Lymphopoiesis wikipedia , lookup
DNA vaccination wikipedia , lookup
Complement system wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Molecular mimicry wikipedia , lookup
Inflammation wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Immune system wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Adaptive immune system wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
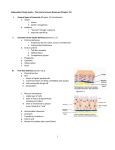
Immunity to infection involves a constant battle between the host defenses and the mutant microbes trying to evolve evasive strategies. Specific acquired responses amplify and enhance innate immune mechanisms. Inflammation revisited • Inflammation is a major defensive reaction initiated by infection or tissue injury. The acute inflammatory response • The mediators released upregulate adhesion molecules such as P-selectin on endothelial cells. These pair with ligands on leukocytes, initially causing rolling of leukocytes along the vessel wall and then passage across the blood vessel up the chemotactic gradient to the site of inflammation. • Under the influence of interleukin-1 (IL-1) and tumor necrosis factor (TNF), ICAM-1 (intercellular adhesion molecule-1) is induced on endothelial cells and binds to integrins on polymorphonuclear neutrophil (PMN) surfaces causing their arrest. • Macrophages and mast cells present in the inflamed tissue are activated and release a variety of mediators, cytokines and chemokines. Endothelial cells themselves may release cytokines and chemokines. • Activated granulocytes and macrophages easily phagocytose organisms coated with antibody and complement proteins. • Cytokines such as transforming growth factor-b (TGFb) and IL-10 are powerful regulators of inflammation and endogenous glucocorticoids. • When tissue is severely traumatized, TGFb may stimulate fibroblasts to lay down collagen forming scar tissue, which is the end result of many inflammatory events. • Inability to eliminate the initiating agent leads to a chronic inflammatory response dominated by macrophages and often forming granulomas. Extracellular bacteria susceptible to killing by phagocytosis and complement • Bacteria try to evade the immune response by surrounding themselves with capsules to avoid phagocytosis, secreting exotoxins which kill phagocytes or impede inflammatory reactions, resisting insertion of the complement membrane attack complex, or by secreting enzymes which destroy C5. • Antibody combats these tricks by neutralizing the toxins, and by overcoming the antiphagocytic nature of the capsules by opsonizing them with immunoglobulin G (IgG) and C3b. • Antigen-presenting cells (APCs) have receptors for microorganisms, such as the Toll-like receptors (TLRs), which when activated lead to the production of proinflammatory cytokines. • Mannose binding lectin (MBL) binds to mannose on bacterial surfaces. In association with MASP-1 and MASP2 it leads to a further pathway of complement activation. • Complement activation is controlled by a number of inhibitory proteins and by various complement receptors. Protection of mucosal surfaces • Defensins are antimicrobial proteins produced by macrophages and mucosal cells. Their production is upregulated by proinflammatory cytokines. • The secretory immune system protects the external mucosal surfaces. Immunoglobulin A inhibits adherence of bacteria and can opsonize them. • Immunoglobulin E bound to mast cells can be found in mucosal tissue. In contact with antigen it will cause degranulation of the mast cells. This initiates release of mediators which generate a local inflammatory reaction. Bacteria which grow in an intracellular habitat • Intracellular bacteria such as tubercle and leprosy bacilli grow within macrophages. • They are killed by cell-mediated immunity (CMI): specifically sensitized T-cells become activated and release interferon g (IFNg) which activates the macrophage to kill the organisms. • When intracellular organisms are not destroyed, a chronic inflammatory reaction will lead to the formation of a macrophage rich granuloma. Immunity to viral infection • Infected cells release type 1 interferons which have antiviral activity. • Natural killer (NK) cells are activated by IFNg and IL-2. They can now attack virally infected cells which have (continued) 126 PART 4—Immunity to infection FURTHER READING Bloom, B. & Zinkernagel, R. (1996) Immunity to infection. Current Opinion in Immunology, 8, 465–6. Brandtzaeg, P. (1995) Basic mechanisms of mucosal immunity: A major adaptive defense system. The Immunologist, 3, 89–96. Finkelman, F.D. & Urban, J.F. (2001) The other side of the coin: The protective role of the Th2 cytokines. Journal of Allergy and Clinical Immunology, 107, 772–80. Ley, K. (2002) Integration of inflammatory signals by rolling neutrophils. Immunological Reviews, 186, 8– 18. Nathan, C. (2002) Points of control in inflammation. Nature, 420, 846–52. Price, D.A., Klenerman, P., Booth, B.L., Phillips, R.E. & Sewell, A.K. (1999) Cytotoxic T lymphocytes, chemokines and antiviral immunity. Immunology Today, 20, 212–16. Rappuoli, R., Pizza, M., Douce, G. & Dougan, G. (1999) Structure and mucosal adjuvanticity of cholera and Escherichia coli heat-labile enterotoxins. Immunology Today, 20, 493– 500. downregulated major histocompatibility complex (MHC) class 1 expression. • Antibody neutralizes free virus and is particularly effective when the virus has to travel through the bloodstream before reaching its final target. • Antibody is important in preventing reinfection. • “Budding” viruses that can invade lateral cells without becoming exposed to antibody are combated by CMI. Infected cells express a processed viral antigen peptide on their surface in association with MHC class I. • Rapid killing of the cell by cytotoxic ab T-cells prevents viral multiplication. • Cytokines produced by CD4+ and CD8+ cells activate APCs and control the replication of virus particles. • Natural infection generates specific antibody and Tcytotoxic (Tc) cells with subsequent long-term protection against reinfection. Immunity to fungi • Fungal infections are common in individuals with neutrophil dysfunction and in patients with defective CMI. Immunity to parasitic infections • Chronic parasitic infection can cause exaggerated immune responses leading to severe tissue injury. • Antibodies are usually effective against the bloodborne t cells. parasitic diseases. • Most parasitic infections stimulate a Th2 response, with IgE production and eosinophilia. These are important in destruction of parasites such as schistosomes, which when coated with IgG or IgE are killed by adherent eosinophils through the mechanism of antibodydependent cellular cytotoxicity (ADCC). • Organisms such as Leishmania spp., Trypanosoma cruzi and Toxoplasma gondii hide from antibodies inside macrophages and use the same strategies as intracellular parasitic bacteria to survive. Like them, they are killed when the macrophages are activated by cytokines produced during CMI responses. • Cytokines produced by Th2 cells are important in the expulsion of gastrointestinal worms and in destroying larval forms. They may also protect against ectoparasites