* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Poster Thomas Sutherland DMMI - Workspace
Cell membrane wikipedia , lookup
Chromatophore wikipedia , lookup
Signal transduction wikipedia , lookup
Endomembrane system wikipedia , lookup
Tissue engineering wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell growth wikipedia , lookup
Cytokinesis wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell culture wikipedia , lookup
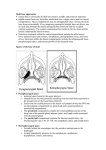
A Model System to Investigate Translocation of Neisseria meningitidis Thomas C. Sutherland & Prof. C. Tang Centre for Molecular Microbiology and Infection, Flowers Building, Imperial College London Meningococcal septicaemia Translocation across bloodbrain barrier 800 600 400 Meningitis 800 600 400 200 200 0 72 0 72 • Current studies limited to expensive, technical tissue explants and culture of cells on impermeable substrates. • The human bronchial epithelial cell line ‘Calu-3’ can be grown into a differentiated epithelium, and has been much used in aerosol medicine studies3, however, it has never been used to study N. meningitidis. Aim • Establish a physiologically relevant cell culture model of the upper respiratory epithelium and use it to investigate translocation of N. meningitidis. Method 1000 Apical Chamber Cell culture insert Porous membrane (1μm pore size) Calu-3 cells Basal Chamber 96 120 98 5.00E+07 4.50E+07 4.00E+07 3.50E+07 3.00E+07 2.50E+07 2.00E+07 1.50E+07 1.00E+07 5.00E+06 0.00E+00 Avg Bacteria per cell (Normalised to input) Avg Bacteria per cell 10 5 AP 0 Chang Calu-3 Cell Type TJ CCM D Monolayer of Calu-3 cells on the cell culture membrane (CCM). Apical (AP)/basal (BA) differentiation, the formation of tight junctions (TJ), desmosomes (D) and microvilli (MV) are clearly all visible. 2 4 6 0 8 2 4 6 8 Time (hrs) siaD::ery/wt 2.0 2.0 1.5 1.5 1.0 1.0 0.5 C The Junctional complex (JC) consisting of tight junctions, the adhesion belt and desmosomes. D • Calu-3 cells have not previously been infected with N. meningitidis. Test infections demonstrate that they undergo a similar pattern of infection to the more studied ‘Chang’ cell line (A). DP TJ E Occludin C 0.0 Desmosome: showing desmoplakins (DP), desmogleins (DG) and intermediate filament (IF) accumulation. 24-25h • Competitive infections, comparing wt N. meningitidis with a capsule mutant (ΔsiaD) (A) and a pilus mutant (ΔpilE) (B). Both mutants show a defect for traversal of the monolayer compared to wt, but the capsule mutant’s defect is greater (average CI after 24hrs of 0.01 compared to for the 0.37 pilus mutant). Conclusions • A protocol to grow Calu-3 cells on cell culture inserts to form a polarised, differentiated, epithelial monolayers has been optimised. • The epithelial monolayers are impermeable to non-invasive bacteria and generate trans-epithelial electrical resistance (TEER) showing that they are confluent and not ‘leaky’. Invasive N. meningitidis is able to penetrate the monolayers. • The pilus mutant shows a competitive disadvantage for translocation compared to wt, a result consistent with a previous study performed in polarised intestinal epithelial cells4. • Future work will involve use of the model to understand the host and pathogen roles in the translocation process. DG Detail of Tight Junctions: TEM shows tight association of cell membranes, while IF staining shows presence of tight junction proteins Occludin and ZO-1. 24-25h • We show for the first time that the capsule mutant has massive translocation defect compared to wt. Previous work has shown that the capsule is essential for intracellular survival5. These data suggest N. meningitidis translocates across the epithelium via an intracellular, rather than paracellular, route. IF IF ZO-1 7-8h • N. meningitidis can infect Calu-3 cells in a manner consistent with other cell lines used in the field. Strain • Type IV Pilus dependant adhesion, previously documented in other cell lines2 is also observed in the Calu-3 cells (B & C). 1.E+00 7-8h MV TJ MC58 1.E+01 AB 15 ΔpilE::kan 1.E+02 0.5 TJ BA 0 No Cells Cells D JC 5 1.E+03 Figure 5 – Translocation mutants A B pilE::kan/wt Figure 3 – Morphology of the cultured cell layers B A Intracellular 10 1.E+04 • The N. meningitidis strain MC58, on the other hand is able to penetrate the monolayer (B). • Coating cell culture inserts with extracellular matrix proteins affects the development of TEER. A 1:1 mixture of laminin and fibronectin is most effective (A). Optimisation culture procedure allows reliable generation of TEER (B). Figure 1 – N. meningitidis infection of Calu-3 cells A 25 B 25 Adherent Adherent 15 1.E+05 • The monolayer is impermeable to non-invasive bacteria (E. coli DH5α) (A). 0.0 20 1.E+07 1.E+06 Time (Hrs) • Trans-epithelial electrical resistance (TEER) develops as tight junctions form and is a good indicator of monolayer polarisation. Unpolarised cells generate TEER readings of 0-100Ω. • Cells are grown on inserts in cell culture plates. This creates basal and apical chambers separated by the differentiated epithelium. Translocation can be analysed by infecting the apical chamber and monitoring the passage of bacteria to the basal chamber. 20 1.E+08 No cells Cells Time (hrs) Time after seeding (hrs) Well of cell culture plate Intracellular 1.E+09 0 122 CI Colonisation of the nasopharynx 1000 Laminin Fibronectin Fibronectin+Laminin cfu/ml in basal chamber TEER(Ω) • Understanding translocation is vital to understanding the disease process. Translocation across Nasopharyngeal Epithelium 1200 CI • Adherence and colonisation is type IV pilus dependant2 but pathway for translocation across the nasopharyngeal epithelium is unknown. cfu/ml in basal chamber • Gram-negative diplococci, harmlessly colonises the nasopharynx of up to 40% of the population1. Figure 4 – Permeability of the monolayer to bacteria A B DH5α Infection MC58 Infection Figure 2 – Optimising the cell culture conditions No Coating A 1400 B 1200 TEER (Ω) Pathogenesis of Neisseria meningitidis Adhesion Belt: (AB) Intracellular and intercellular attachment proteins visible. • TEM (cross sections, 120 hrs post seeding) and Confocal microscopy (monolayers viewed from above, 120 hrs post seeding) demonstrate that the cultured cells reliably form confluent, polarised monolayers with all the morphological features of a polarised epithelium. References 1. K. A. Cartwright et al., Epidemiol. Infect. 99: 591 (1987). 2. X. Nassif, C. Pujol, P. Morand, E. Eugene, Mol. Microbiol. 32: 1124 (1999). 3. K. Foster, M. Avery, M. Yazdanian, K. Audus, Int. J. Pharm. 208: 1 (2000). 4. C. Pujol, E. Eugene, L. de Saint Martin & X. Nassif, Infect. Immun. 65: 4836 (1997). 5. M. Spinosa, C. Progida, A. Tala, L. Cogli, P. Alifano, C. Bucci, Infect. Immun. 75:3594 (2007). Acknowledgements Special thanks must go to Mike Hollinshead from the Henry Wellcome imaging suite on the St Mary’s Campus for all his help with the Transmission Electron Microscopy.