* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The C-terminal end of R-Ras contains a focal adhesion targeting
Cell growth wikipedia , lookup
Tissue engineering wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell membrane wikipedia , lookup
Cell culture wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cytokinesis wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cell encapsulation wikipedia , lookup
Signal transduction wikipedia , lookup
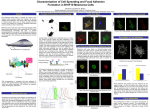
Research Article 3729 The C-terminal end of R-Ras contains a focal adhesion targeting signal Johanna Furuhjelm and Johan Peränen* Institute of Biotechnology, Program in Cellular Biotechnology, PO Box 56 (Viikinkaari 9), FIN-00014 University of Helsinki, Finland *Author for correspondence (e-mail: [email protected]) Accepted 28 May 2003 Journal of Cell Science 116, 3729-3738 © 2003 The Company of Biologists Ltd doi:10.1242/jcs.00689 Summary R-Ras promotes cell adhesion and activation of integrins through a process that is yet unknown. We show here that active R-Ras (38V) promotes the formation of focal adhesions and a spread cell shape. By contrast, the dominant-negative mutant of R-Ras (43N) reduces the number of focal adhesions, leading to the formation of refractile cells. In adherent cells wild-type R-Ras, activated (38V) R-Ras and endogeous R-Ras were preferentially targeted to focal adhesions, whereas the dominant-negative mutant (43N) of R-Ras was excluded from these structures. Activated mutants of H-Ras and K-Ras were not found in focal adhesions. We dissected R-Ras to find out the determinants that are important for the targeting process. The outermost region in the N-terminus of R-Ras, as well as the intact proline-rich sequence in the C-terminus of RRas that mediates binding to Nck, were not essential. Mutating the potential palmitoylation site (C213A) of RRas results in depalmitoylation and accumulation of R-Ras in the Golgi. Using H-Ras/R-Ras, R-Ras/H-Ras and RRas/K-Ras hybrid molecules we showed that the C-termini (175-218 amino acids) of R-Ras contains the signal for focal adhesions targeting. Exchanging the hypervariable region of H-Ras to R-Ras inhibited the targeting of R-Ras to focal adhesions, whereas H-Ras obtained the ability to localize to focal adhesions after receiving the hypervariable region of R-Ras. This indicates that R-Ras targeting is mediated both by the nucleotide binding status as well as through a specific region in the C-terminus of R-Ras. These results indicate that targeting and activation of R-Ras are linked processes in the formation of focal adhesions. Introduction Ras proteins are important regulators of cell differentiation and growth that localize to the inner surface of the plasma membrane (Crespo and Leon, 2000). Ras acts as a molecular switch, converting signals from the cell surface to the nucleus. In human cells there are four closely related Ras proteins: HRas, N-Ras, K-RasA and K-RasB. Although these proteins interact with the same set of effectors in vitro, they activate them with different efficiency (Voice et al., 1999). They also show a variable ability to induce cell transformation and cell motility (Voice et al., 1999). In addition, it has become clear that K-Ras and H-Ras operate in different microdomains at the plasma membrane (Prior et al., 2001; Prior and Hancock, 2001). H-Ras is found in lipid rafts and segregates into the bulk membrane after activation, whereas K-Ras is located in the bulk membrane, irrespective of bound nucleotide. The difference in location is at least partly due to the hypervariable region, where less than 10-15% of the residues are identical among the four Ras proteins (Prior et al., 2001). R-Ras shows 55% identity with Ras, and its minimal effector region is identical to that of Ras, but R-Ras contains a 26 amino acid (aa) extension in its N-terminus. Many Ras effectors and some exchange factors interact with R-Ras, but R-Ras shows a much lower ability to transform cells compared with Ras (Cox et al., 1994). However, R-Ras regulates cell adhesion, spreading and phagocytosis by activating integrins (Zhang et al., 1996; Berrier et al., 2000; Self et al., 2001), and it has also been shown to antagonize Ras/Raf-initiated integrin suppression (Sethi et al., 1999). How R-Ras activates integrins is not known, but its effector loop and prenylation site, as well as the proline-rich sequence in the hypervariable region of RRas, are essential for this activation process (Oertli et al., 2000; Wang et al., 2000). The proline-rich region has been shown to bind the adaptor protein Nck, which is known to interact with proteins accumulating in focal adhesions (Wang et al., 2000). Furthermore, the Eph receptor tyrosine kinase, EphB2, phosphorylates a tyrosine residue in the effector region of RRas, leading to suppression of R-Ras-mediated adhesion (Zou et al., 1999). A similar relationship has also been found to exist between activated Src and R-Ras (Zou et al., 2002). Focal adhesions (FA) are specialized signalling platforms on the cell surface that mediate cell-matrix interactions via integrins, which are associated with different cytoskeletal proteins (Sastry and Burridge, 2000). FAs are dynamic structures that assemble and disassemble when cells migrate or divide. This assembly/disassembly process is regulated by the Rho GTPases (Kaibuchi et al., 1999). Activation of RhoA promotes the assembly of large focal adhesions through increased contractility, whereas Rac1 induces the formation of small adhesions called focal complexes at the leading edge of migrating cells (Nobes and Hall, 1995). The turnover of FAs is regulated by Ras (Nobes and Hall, 1999). However, FAs may not be the only platforms that mediate cell signalling. Recent studies indicate that microdomains called lipid rafts may also Key words: GTPase, R-Ras, Targeting, Focal adhesion 3730 Journal of Cell Science 116 (18) participate in signal transduction (Simons and Toomre, 2000). Whether the lipid rafts have a direct role in processes mediating cell adhesion is still uncertain (Pande, 2000). To better understand the role of R-Ras in cell adhesion we decided to study its targeting to the cell surface. We show here that R-Ras is preferentially targeted to focal adhesions and that this targeting process is dependent on the nucleotide state of R-Ras. Only GTP-bound R-Ras is associated with focal adhesions, whereas the GDP form is excluded from these structures. The hypervariable region of R-Ras was sufficient in targeting another -Ras protein to focal adhesions, indicating that this region contains the targeting signal. Finally, we show that the targeting of R-Ras and the integrity of focal adhesions is dependent on the cholesterol content of the plasma membrane. Our data underscore the importance of specific localization of Ras molecules on the plasma membrane in mediating cell signalling. Cells transfection, labelling and cholesterol depletion Hela cells were grown overnight on collagen-coated (30 µg/ml) coverslips and transiently transfected with constructs expressing RRas, Rac and their corresponding mutants by Fugene 6 according to the manufacturer (Roche) (Peränen and Furuhjelm, 2001). Equal molarity of constructs was used in double transfection studies. Labelling of pEGFP-H-Ras61L-, pEGFP-R-Ras38V- and pEGFP- Materials and Methods Constructs Plasmid pEXV-Ras-wt (a gift from A. Hall) was used to prepare RRas-38V and R-Ras-43N mutants by PCR mutagenesis, and these were cloned into pGEM-3 (Promega, Madison, WI) (Peränen et al., 1996). Two mutations, P202A and P203A, were made in the hypervariable region of R-Ras-38V in the pGEM-R-Ras-38V plasmid. A similar approach was used to create the S172P and C213A mutations. The open reading frames (ORFs) from the pGEM constructs of R-Raswt, R-Ras38V, R-Ras-43N, R-Ras38V/C213A and R-Ras-38V/S172P were cut out and cloned into either pcDNA4/TO (Invitrogen, Carlsbad, CA) or pEGFP-C1A (Clontech, Palo Alto, CA) (Peränen and Furuhjelm, 2001). A fragment corresponding to the hypervariable region of R-Ras (191-218 aa) was cloned into pEGFPC1A to obtain pEGFP-HVR. Plasmid pEGFP-R-Ras38V-deltaNT was created by cloning a PCR fragment, corresponding to the 29-218 aa in R-Ras, into pEGFP-C1A. Plasmid pEGFP-R-Ras38V-dC contained R-Ras38V ORF missing the last six amino acids from the C-termini (213-218). Plasmid pEGFP-R-Ras38V contained R-Ras38 that was deleted of its 176-212 amino acids but retained the last six amino acids (213-218). H-Ras and K-Ras were obtained by PCR from human HeLa cDNA, and H-Ras61L and K-Ras12V were created by PCRbased mutagenesis. H-Ras61L/R-Ras38V, R-Ras38V/H-Ras61L and R-Ras38V/K-Ras12V chimeras were constructed by splice overlap mutagenesis. These genes were ultimately cloned into pEGFP-C1A. Plasmids pEXV-Rac12V and pEXV-Rac17N (gifts from A. Hall) were used to excise the corresponding Rac1 mutants to reclone them to pEGFP-C1A and pEGFP-N1 (Clontech). The human Arf6 reading frame was amplified by PCR from HeLa cDNA and cloned into pEGFP-N1 or pcDNA4/TO; the Arf6-27N and Arf6-67L mutants were made by site-specific PCR mutagenesis as described earlier (Peränen et al., 1996). All constructs based on PCR were verified by DNA sequencing. Details of the constructs are available on request. Western blot For western blot analysis HeLa cells were grown overnight on two 6 cm plates and transiently transfected with pEGFP-R-Raswt, pEGFPR-Ras38V or pEGFP-R-Ras43N using Fugene 6 according to the manufacturer (Roche Diagnostics, Mannheim, Germany). After 20 hours the cells, which were about 80% confluent, were lysed by SDSPAGE (sodium dodecyl sulphate-polyacrylamide gel electrophoresis) sample buffer and the DNA was disrupted by passing it through a needle. The presence of R-Ras was detected by western blot using anti-R-Ras (Santa Cruz Biotechnology, Santa Cruz, CA) as previously described (Peränen et al., 1996). Fig. 1. R-Ras uses endomembranes to reach the plasma membrane. Hela (A-C) and Cos-7 (D-F) cells were transfected with vectors pEGFP-R-Raswt (A,B) and pEGFP-R-Ras43N (C,D), or double transfected with pEGFP-R-Ras43N and pcDNA4/TO-Arf6-27N (E,F). EGFP-R-Raswt colocalized with a p115 Golgi marker (arrow) and was also seen on the nuclear envelope (arrowhead). In addition to these organelles, EGFP-R-Ras43N was found on vesicular (C) and vacuolar (D) structures, which colocalized with Arf6-27N (arrowheads) (E,F). HeLa cells were transfected with pEGFP-RRaswt (wt), pEGFP-R-Ras38V (38V), pEGFP-R-Ras43N (43N) and pEGFP-C1A, and total cell extracts were analysed by western blot (G). The positions of molecular size markers are indicated. Targeting of R-Ras to focal adhesions Rras38V/213A-transfected HeLa cells with [9,10(n)-3H]palmitic acid (Amersham Biosciences Europe) at 200 µCi/ml in modified Eagle’s medium plus 5% dialysed bovine serum for 4 hours. Immunoprecipitation of the indicated Ras molecules from the labelled cells was done by H-Ras- and R-Ras-specific antibodies as described earlier (Peränen and Furuhjelm, 2001). The immunoprecipates were analysed by SDS-PAGE and fluorography. Cholesterol depletion of HeLa cells with 0.5-1% β-methylcyclodextrin (MβCD) for 30-60 minutes was carried out as described previously (Parton and Hancock, 2001). Cholesterol/MβCD inclusion complexes were added to 3731 cholesterol-depleted cells or to cells grown in serum (Parton and Hancock, 2001). Cell spreading Subconfluent Hela cells were transfected overnight with indicated EGFP-R-Ras constructs. They were then harvested with 0.5 mM EDTA in phosphate-buffered saline (PBS), resuspended in MEM containing fatty-acid-free bovine serum albumin (5 mg/ml), and the cells were counted. An appropriate number of cells were then plated onto collagen- (30 µg/ml) coated cover slips. After 60 minutes at 37oC cells were fixed with paraformaldehyde and stained for vinculin. Spread versus unspread EGFP-positive cells were counted. Immunocytochemistry Transfected C2C12 or HeLa cells were processed for immunofluorescence or confocal microscopy as previously described (Peränen et al., 1996; Peränen and Furuhjelm, 2001). Antibodies used were anti-Arf6 (NeoMarkers, Fremont, CA), anti-caveolin (BD Biosciences, San Diego, CA), anti-β1 integrin (BRL/GIBCO, Gaitersburg, MD), anti-paxillin (Trans lab), anti-phospho-caveolin (Trans lab), anti-p115 (Trans lab), anti-R-Ras (Santa Cruz), anti-talin (Sigma), anti-vinculin (Sigma). Goat anti-rabbit IgG-lissamine and goat anti-mouse IgG lissamine were from Jackson Immunoresearch. Results R-Ras uses the endomembrane transport route To investigate the targeting of R-Ras, we constructed constitutive active (38V) and dominant-negative (43N) mutants of R-Ras and tagged them to EGFP. Western blot analysis of Hela cell extracts harbouring these constructs showed the presence of a 55 kDa protein, as well as endogenous R-Ras (Fig. 1G). The constructs, and corresponding constructs of the original wild-type R-Ras, were then transfected into HeLa cells and processed for immunofluorescence and confocal microscopy. Each of the RRas proteins localized to a perinuclear region, to the Golgi and to the plasma membrane (Figs 1, 2). Identical results were obtained for untagged Ras constructs (not shown). The subcellular localization of R-Ras was very similar to that found for H-Ras and N-Ras, suggesting that R-Ras uses an analogous endomembrane trafficking route (Choy et al., 1999). In addition, we found EGFP-R-Ras43N on numerous vesicles in HeLa cells, and on vacuoles in COS cells (Fig. 1C,D). The EGFP-R-Ras43N vesicles were not positive for such known organelle markers as Rab4, Rab5, Rab6 and Rab11 (not shown). However, we observed that EGFP-R-Ras43N Fig. 2. Plasma membrane localization of R-Ras molecules. HeLa cells transiently expressing EGFP-R-Raswt (A), EGFP-R-Ras38V (B), EGFP-R-Ras43N (C) and EGFP (D) were fixed and analysed by fluorescence microscopy. Note that EGFP-R-Raswt and EGFP-RRas38V are found in adhesion-like structures, whereas EGFP-RRas43N and EGFP are not. Localization of endogenous R-Ras (E) to vinculin (F) containing focal adhesions in untransfected HeLa were seen using specific antibodies against R-Ras and vinculin. EGFP-RRas38V was also detected in focal adhesions in C2C12 cells (G,H). Arrowheads indicate focal adhesion-like localization. Bars, 10 µm. (I) The percentage of cells containing R-Ras in focal adhesions was calculated for R-Raswt, R-Ras38V and R-Ras43N. Values are means ± s.d. from three independent experiments in which >100 cells were scored per condition. 3732 Journal of Cell Science 116 (18) Fig. 3. R-Ras in focal adhesions. Cells were transfected with pEGFP-R-Ras38V (R-Ras38V), and pEGFP-R-Ras43N (R-Ras43N) fixed, stained for vinculin and analysed by confocal microscopy. Note that R-Ras43N does not colocalize with vinculin, whereas R-Ras38V shows a strong colocalization. In R-Ras-38V/Rac1-12V the pCDNA4-TO-R-Ras38V vector was cotransfected with pEGFP-Rac12V and stained with an antibody against R-Ras. Arrowheads indicate colocalization of R-Ras and EGFP-Rac1 to focal adhesions. Bars, 10 µm. colocalized with Arf6, especially with the Arf6-27N mutant on vesicular structures (Fig. 1E,F), indicating that R-Ras could be internalized to a recycle compartment (Brown et al., 2001). GTP-dependent targeting of R-Ras to focal adhesions The plasma membrane is considered to be the main platform for Ras-mediated signalling, where R-Ras is thought to regulate adhesion and integrin activation (Zhang et al., 1996; Sethi et al., 1999). Interestingly, both EGFP-R-Raswt and EGFP-R-Ras38V were localized to focal adhesion-like structures in HeLa cells, whereas EGFP-R-Ras43N was evenly distributed on the plasma membrane (Fig. 2A-F). EGFP-RRas38V showed the strongest staining intensity in focal adhesions. Quantification of cells harbouring the abovementioned constructs showed that 84% of the R-Ras38Vexpressing cells contained R-Ras38V in focal adhesions, whereas the value for EGFP-R-Raswt was 40% (Fig. 2I). By contrast, only 0.5% of cells expressing EGFP-R-Ras43N contained R-Ras-positive focal adhesions, suggesting that the GTP-bound form of R-Ras is preferentially targeted to focal adhesions. When R-Ras was expressed in excess its localization to the focal adhesions was more difficult to observe due to strong staining of the surrounding plasma membrane, possibly indicating that the binding of R-Ras to focal adhesions was saturated (data not shown). Identical results were obtained with untagged R-Ras constructs in combination with anti-R-Ras antibodies (Fig. 3). R-Ras-38V was also observed in C2C12 cells (Fig. 2G,H), therefore its localization to focal adhesions was not restricted to HeLa cells. However, in the HT1080 fibrosarcoma cell line and COS-7 cells EGFP-R-Ras38V was not associated with focal adhesions (not shown). HT1080 cells contain a mutant N-ras allele that mediates a transformed phenotype, including disorganized actin and poor adherence, and these features may counteract the targeting of R-Ras to focal adhesions (Marshall et al., 1982). This indicates that the localization of R-Ras to focal adhesions is typical for strongly adherent cells, like HeLa and C2C12. Furthermore, we also showed that endogenous R-Ras in HeLa cells is localized to focal adhesions that are positive for vinculin (Fig. 2E,F). EGFP alone did not localize to focal adhesions, but was preferentially found in the nucleus (Fig. 2D). To characterize the identity of R-Ras-containing focal adhesions we stained EGFP-Ras38V-expressing HeLa cells with known focal adhesion markers. Vinculin colocalized nicely with EGFP-Ras38V when analysed by confocal microscopy (Fig. 3). This was also true for β1-integrin, talin and paxillin (data not shown). By contrast, EGFP-R-Ras43N did not colocalize with these markers (Fig. 3). It has recently been shown that Rac12V colocalizes with p21-activated protein kinase, α-PAK, to focal adhesions of HeLa cells (Manser et al., 1997). Likewise, we found that EGFP-Rac12V localized with R-Ras38V in focal adhesions, indicating that there may be a functional link between Rac1 and R-Ras (Fig. 3). Targeting of R-Ras to focal adhesions 3733 Fig. 4. R-Ras modulates focal adhesion formation and cell spreading. Hela cells grown on collagen-coated glass coverslips were transfected overnight with pEGFP-Raswt (A,D), pEGFP-R-Ras38V (B, E) and pEGFP-RRas43N (C,F). Note that the R-Raswt cell contains distal focal adhesions, the R-Ras38V cell contains numerous large focal adhesions and the R-Ras43V cell contains few small adhesions. R-Raswt cells were often elongated (D), RRas38V cells (E) well spread and R-Ras43N cells (F) retracted with filopodia-like structures. The number of focal adhesions was calculated from above transfected cells, 15 cells/construct/ experiment (J). Results shown are the mean ± s.e.m. of three experiments. Cells expressing EGFP-Raswt, EGFP-R-Ras38V and pEGFP-RRas43N were also plated on collagen-coated coverslips for 60 minutes for estimation of cell spreading (K). 30 cells were counted for each construct and experiment. Results shown are the mean ± s.e.m. of three experiments. Typical spread cells are shown for corresponding construct in G, H and I. Note the numerous large focal adhesions in EGFP-R-Ras38V cells and the few small in EGFP-R-Ras43N cells. Bars, 10 µm. Focal adhesions formation is linked to R-Ras activity R-Ras is known to regulate cell adhesion. Thus, we next studied whether R-Ras influences the structure and number of focal adhesions in HeLa cells. Cells were transfected overnight with constructs encoding EGFP-R-Raswt, EGFP-R-Ras38V or EGFP-R-Ras43N (Fig 4). Cells expressing EGFP-R-Raswt were often elongated and contained focal adhesions in the distal regions of the cell. When cells expressed EGFP-R-Ras38 they were symmetrical and spread, and they contained large focal adhesions that were located nearer the center of the cell. By contrast, cells expressing EGFP-R-Ras43N were contracted and often contained filopodia-like structures. Moreover, the focal adhesions located distally and were very small compared with those found in EGFP-R-Ras38-expressing cells. We next quantified the number of focal adhesions in cells expressing these EGFP-R-Ras constructs (Fig. 4J). We found that RRas38V-expressing cells contained more focal adhesions than did R-Raswt, whereas R-Ras43-expressing cells had fewer adhesions than R-Raswt. This suggests that active R-Ras promotes the formation of focal adhesions, whereas R-R-Ras inhibits their formation. The same EGFP-R-Ras constructs were also used to test cell spreading (Fig. 4K). EGFP-R-Raswt and EGFP-R-Ras38V cells spread with an equal efficiency, whereas EGFP-R-Ras43N expression led to fewer spread cells. The difference in cell spreading correlated with the formation of focal adhesions. EGFP-R-Ras38N cells were symmetrical with large focal adhesions, whereas EGFP-R-Raswt cells had adhesions more peripherally. By contrast, EGFP-R-Ras43Nexpressing cells contained very few and tiny focal adhesions. In summary, our results show that R-Ras is essential for the formation of focal adhesions, and confirm previous studies that R-Ras controls cell spreading (Berrier et al., 2000; Zhang et al., 1996). Potential targeting signals in R-Ras R-Ras contains an extended N-terminus compared with other Ras molecules. We deleted this N-terminal region (1-28 aa) and expressed the molecule, EGFP-R-Ras38V-NT, in HeLa cells. Because EGFP-R-Ras38V-NT was nicely localized to focal 3734 Journal of Cell Science 116 (18) Fig. 5. R-Ras constructs and R-Ras palmitoylation. (A) GFP constructs used were wild-type R-Ras (pEGFP-R-Raswt), constitutively active R-Ras38V (pEGFP-R-Ras38V), dominantnegative R-Ras43 (pEGFP-R-Ras43N), constitutively active R-Ras deleted of the first 28 amino acids (pEGFP-R-Ras38V-NT), constitutively active R-Ras38V deleted of its last six amino acids (pEGFP-R-Ras38VdC), constitutively active R-Ras38V deleted of its HVR (175-212) but containing the last six amino acids (212-218) (pEGFP-R-Ras38V-dHVR), constitutively active R-Ras38V-2PA with two proline substituted on P202A and P203A (pEGFP-RRas38V-2PA), constitutively active R-Ras38V with a mutated palmitoylation site C213A (pEGFP-R-Ras38V-CA), constitutively active R-Ras38V with both palmitoylation and nucleotide destabilizing mutations C213A/S172P (pEGFP-R-Ras38V-SP) and the hypervariable region encoding 191-218aa of R-Ras (pEGFPHVR). (B) HeLa cells were transfected overnight with the following constructs: pEGFP-H-Ras61L (1), pEGFP-R-Ras38V-CA (2) and pEGFP-R-Ras38V (3). The cells were then labelled with [3H]palmitic acid for 4 hours, immunoprecipitated and analysed by SDS-PAGE. Note that the construct encoding EGFP-R-Ras with a mutated palmitoylation site (C213A; lane 2) is not labelled with palmitate, whereas EGFP-H-Ras61L (lane 1) is strongly labelled, and EGFP-R-Ras38V (lane 3) to a lesser extent. targeting R-Ras to focal adhesions. To test this hypothesis we fused the hypervariable region (HVR: 191-218 aa) of R-Ras to EGFP (Fig. 5A). This EGFP-HVR was efficiently transported to the plasma membrane via Golgi. However, it was not targeted to focal adhesions; instead, it showed a uniform distribution at the plasma membrane, indicating that this part of the hypervariable region is essential for membrane targeting but insufficient for localization of R-Ras to focal adhesions (Fig. 6C,D). We also made a construct encoding a protein (EGFP-R-Ras38V-dHVR) that lacked the whole HVR (175212 aa) but which contained the six outermost amino acids needed for lipid modification. This protein was trapped in the Golgi and on small vesicles, indicating that HVR is essential for proper transport to the plasma membrane (Fig. 6E,F). The proline-rich sequence in the hypervariable region binds a SH3 domain of Nck, indicating that it might contribute to the targeting mechanism of R-Ras (Wang et al., 2000). However, the introduction of mutations (P202A, P203A) in the prolinerich sequence of R-Ras38V did not inhibit targeting of RRas38V to focal adhesions (Fig. 5A; Fig. 6G,H). R-Ras is palmitoylated and contains a potential palmitoylation site (C213) upstream of the CAAX box (Lowe and Goeddel, 1987; Schmittberger and Waldmann, 1999). We showed that EGFP-R-Ras38V is labelled with palmitate, whereas EGFP-R-Ras38V/C213A, which contains a mutation in the potential palmitoylation site did not incorporate palmitate, suggesting that cysteine 213 is the target for palmitoylation in R-Ras (Fig. 5A,B). Moreover, EGFP-RRas38V/C213A showed an increased accumulation in Golgi and consequently a decreased appearance on the plasma membrane when compared with the other R-Ras mutants (Fig. 6I,J). Such a Golgi retention has also been observed when the palmitoylation sites of H-Ras and N-Ras are mutated (Choy et al., 1999). EGFP-R-Ras38V incorporates far less palmitate than EGFP-H-Ras61L; this can not be explained simply by the fact that H-Ras contains two palmitoylation sites but R-Ras contains only one (Fig. 5B). Both are labelled equally well by methionine when analysed by immunoprecipitation (not shown). One possibility is that the turnover rate of palmitate is different for these two Ras molecules. The C213A mutation did not inhibit R-Ras38V association with focal adhesions (not shown). The introduction of an additional mutation (S173P), which probably destabilizes nucleotide binding, into EGFP-RRas38V/C213A led to retention of R-Ras in the endoplasmic reticulum (ER), suggesting that proper nucleotide binding or protein folding is needed for transport from the ER (Fig. 5A; Fig. 6K,L). Finally, deletion of both the CAAX box and the palmitoylation site resulted in the accumulation of the molecule in the cytoplasm and nucleus, suggesting that R-Ras can not associate with focal adhesions when it is not bound to the membrane (Fig. 5A; Fig. 6M,N). Taken together, our results show that the hypervariable region of R-Ras is crucial for membrane targeting and transport. adhesions it is unlikely that this region is important for targeting (Fig. 6A,B). The hypervariable region of small GTPases has been implicated in membrane-specific targeting processes (Choy et al., 1999; Chavrier et al., 1991; Michaelson et al., 2001). This raised the possibility that the hypervariable region, including the outermost C-terminus, is responsible for Dissecting the R-Ras-specific targeting signal by using R-Ras/H-ras/K-Ras hybrid molecules H-Ras and K-Ras are known to localize to different subdomains on the plasma membrane (Prior and Hancock, 2001). We fused H-Ras61L and K-Ras12V to EGFP, and expressed them in HeLa cells. In contrast to EGFP-R-Ras, Targeting of R-Ras to focal adhesions neither EGFP-H-Ras61L nor EGFP-K-Ras12V localized to focal adhesions (Fig. 7B). However, both proteins induced the 3735 formation of lamellipodia and ruffles, which are typical for transformed cells. Moreover, their expression resulted in a decrease in the number and size of the focal adhesions. To see whether the HVR of R-Ras is essential for the targeting process, we replaced the 175-218 aa region from R-Ras38V with the corresponding regions (148-189 aa) from H-Ras or from K-Ras (147-188 aa) (Fig. 7A). The hybrid proteins EGFP-R-Ras38V/H-RasC and EGFP-R-Ras38V were expressed in HeLa cells. Neither proteins were localized to focal adhesions but were uniformly distributed at the plasma membrane (Fig. 7B). In addition, both had a phenotype that more resembled H-Ras61L and K-Ras12V. Because the 175218 aa region of R-Ras seemed to be essential for focal adhesion targeting we reasoned that this region could also function in H-Ras as a targeting signal. Thus, we made an HRas61L/R-RasC chimera, which was expressed in HeLa cells (Fig. 7A). This chimera, which is mainly composed of H-Ras, was localized to vinculin-containing focal adhesions, indicating that the 175-218 aa of R-Ras contains the essential elements for focal adhesion targeting (Fig. 7B). Focal adhesions are sensitive to cholesterol depletion It has been shown recently that H-Ras resides in cholesterolrich lipid rafts and caveolae on the plasma membrane, and that activation of H-Ras leads to its segregation from rafts (Prior et al., 2001; Prior and Hancock, 2001). K-Ras, by contrast, is localized predominantly to the disordered plasma membrane (Prior et al., 2001). Caveolin, the main component of caveolae, is known to participate in integrin-mediated adhesion and Ras signalling (Parton and Hancock, 2001; Wei et al., 1999; Roy et al., 1999). Thus, we stained EGFP-R-Ras38V-expressing cells with anti-caveolin and anti-phospho-caveolin recognizing caveolin phosphorylated on tyrosine 14 (Lee et al., 2000). Caveolin was found in patches over the cell surface and along the cell margins, but there was no colocalization with EGFPR-Ras38V (data not shown). By contrast, phospho-caveolin colocalized nicely with EGFP-R-Ras38V in focal adhesions (Fig. 8A,B). To investigate whether the localization of RRas38V to focal adhesions is dependent on cholesterol-rich subdomains, we depleted serum-starved cells expressing EGFP-R-Ras-38V with 0.5-1% β-methylcyclodextrin for 30 minutes. This resulted in the smooth distribution of EGFP-RRas-38V on the plasma membrane and redistribution of phospho-caveolin to small dot-like structures that no longer colocalized with EGFP-R-Ras38V (Fig. 8E,F). This was associated with a simultaneous decrease in the size and number of focal adhesions. Repletion of cholesterol depleted cells with cholesterol/CD inclusion complexes for 30-60 minutes resulted in reformation of focal adhesions and retargeting of both EGFP-R-Ras38V and phospho-caveolin (Fig. 8G,H). Finally, cholesterol replenishment on cells that had not been serum Fig. 6. Determinants affecting R-Ras targeting. HeLa cells were transiently transfected with pEGFP-R-Ras38V-NT (A,B), pEGFPHVR (C,D), pEGFP-R-Ras38V-dHVR (E,F), pEGFP-R-Ras38V-2PA (G,H), pEGFP-R-Ras38V-CA (I,J), pEGFP-R-Ras38V-SP (K,L) and pEGFP-R-Ras38V-dC (M,N), fixed and stained with anti-vinculin. Observe the absence of EGFP-HVR from vinculin-containing adhesions. Note the accumulation of R-Ras38V-dHVR, R-Ras38VCA in Golgi and R-Ras38V-SP in ER. EGFP-R-Ras38V-dC that lacks signals for lipid modifications is found in the nucleus. 3736 Journal of Cell Science 116 (18) starved showed normal focal localization for both EGFP-RRas-38V and phospho-caveolin (Fig. 8C,D). We conclude that the integrity of focal adhesions and the localization of EGFP- A G38V 218 174 pEGFP-R-Ras38V GFP Q61L 147 189 147 188 pEGFP-H-Ras61L GFP G12V pEGFP-K-Ras12V GFP G38V 174 GFP pEGFP-R-Ras38V/H-RasHVR 148 Q61L 189 147 pEGFP-H-Ras61L/R-RasHVR GFP 175 G38V 218 174 pEGFP-R-Ras38V/K-RasHVR GFP 148 188 R-Ras38V and phospo-caveolin to adhesions are dependent on the cholesterol content of the plasma membrane. Discussion We showed here that R-Ras localizes to focal adhesions on the plasma membrane. The localization is determined by the GTP/GDP state, so that only R-Ras-GTP is recruited to focal adhesions. R-Ras-GTP has been shown to enhance both cell adhesion and cell spreading (Zhang et al., 1996; Berrier et al., 2000), and this is supported by our study. We showed that RRas also regulates the formation, number and size of focal adhesions. Thus, it is likely that the targeting of R-Ras-GTP to focal adhesions is functionally linked to an increase in adhesion and spreading. To unravel the targeting signal of R-Ras we constructed different R-Ras mutants. We showed that the N-terminus present in R-Ras is not necessary for the targeting process. Although mutations in the proline-rich sequence of R-Ras38V suppresses cell attachment, the mutants are still more potent than R-Raswt in inducing cell attachment (Wang et al., 2000). Because the mutations in this sequence did not affect R-Ras targeting, the sequence must have other functions that are related to the binding of Nck (Wang et al., 2000). One possibility is that Nck could mediate a cross-talk between RRas and Rac1, because it binds PAK, which is known to interact with Rac1 (Manser et al., 1994; Manser et al., 1997). Moreover, we showed that R-Ras and Rac1 colocalize in focal adhesions. Such a cross-talk could be important in amplifying signals that mediate cell adhesion and spreading. We showed that lipid modification is essential for R-Rasspecific targeting, because R-Ras lacking these signals is found in the cytoplasma and nucleus. In addition, we showed for the first time that amino acid C213 of R-Ras is the most probable attachment site for palmitic acid. When this site was mutated, R-Ras accumulated in the Golgi. This was also the case when the hypervariable region was deleted. Together, this suggests that palmitoylation and the HVR region are important for the transport of R-Ras by endomembranes to the plasma membrane. Fig. 7. Chimeric Ras constructs unravelled the R-Ras-specific focal adhesion targeting signal. (A) Activated forms of H-Ras (61L), K-Ras (12V) and R-Ras (38V), as well as indicated hybrids, were all fused to EGFP. The R-Ras38V/H-RasHVR contained the first 1-174 amino acids from R-Ras38V and the 148-189 amino acids from the C-termini of H-Ras12V. The H-Ras12V/R-RasHVR contained the Ntermini 1-147 amino acids from H-Ras61L and the 175-218 amino acids from the C-termini of R-Ras38V. The R-Ras38V/K-RasHVR hybrid contained the 1-174 amino acids from the N-termini of RRas38V and the 148-188 amino acids from the C-termini of KRas12V. (B) Hela cells were transfected overnight with pEGFP-HRas61L (a,b), pEGFP-K-Ras12V (c,d), pEGFP-R-Ras38V/HRasHVR (e,f) pEGFP-R-Ras38V/K-RasHVR (g,h), and pEGFP-HRas61L/R-RasHVR (i,j), fixed and stained for vinculin. Note that neither K-Ras12V nor H-Ras61L was found in focal adhesions. However, both showed typical ruffle and lamellipodia structures and usually a reduced number of focal adhesions. Replacing the R-Ras specific HVR with corresponding regions from H-Ras61L and KRas12V inhibited R-Ras targeting (e-h). By contrast, adding the HVR from R-Ras to H-Ras61L led to focal adhesion targeting (i,j). Arrowheads show H-Ras61L/R-RasHVR in focal adhesions. Targeting of R-Ras to focal adhesions Fig. 8. Cholesterol-dependent localisation of R-Ras to focal adhesion. Hela cells transfected with pEGFP-R-Ras38V were grown over night in serum-free (K) medium (A,B). Cholesterol replenishment (CD/Chol) was carried out for 30 minutes on EGFPR-Ras38V expressing cells grown overnight in the presence of serum (C,D). Some serum-starved cells were depleted of cholesterol by adding 0.5% β-methylcyclodextrin (MβCD) for 30 minutes (E,F). (G,H) Cholesterol replenishment of cholesterol depleted cells (CDCD/Chol) for 30 minutes. EGFP-R-Ras38V expressing cells (A,C,E,G) were fixed with paraformaldehyde, permeabilized with 0.1% Triton-X-100 and stained with an antibody against phosphocaveolin (P-Caveolin) (B,D,F,H). Bars, 10 µm. Deletions and mutations might have pronounced effects on the function of different proteins, making it difficult for us to find targeting signals. R-Ras is closely related to H-Ras and K-Ras, which makes it possible to switch regions between these molecules without making gross changes in the protein architecture. However, H-Ras and R-Ras have opposing effects on integrin activation. R-Ras promotes integrin activation, whereas H-Ras suppresses integrin activation (Zhang et al., 1996; Hughes et al., 1997). When the hypervariable region of R-Ras (aa 175-218) was replaced by the corresponding region (aa 147-189) of H-Ras the targeting of R-Ras to focal adhesions was inhibited. Interestingly, an identical replacement between R-Ras and H-Ras was recently shown to suppress R-Ras-mediated integrin activation (Hughes et al., 2002). The hypervariable region of K-Ras also 3737 inhibited the targeting of R-Ras. Furthermore, when the hypervariable region of H-Ras (aa 147-189) was replaced by the corresponding region (aa 175-218) of R-Ras, the H-Ras molecule was targeted to focal adhesions, showeing that the hypervariable region of R-Ras contains a focal adhesionspecific targeting signal. Recently, a similar construct was shown to confer R-Ras specificity to H-Ras (Hansen et al., 2002). Taken together, this suggests that the hypervariable region of R-Ras is important for both focal adhesion targeting and integrin activation, and that these two processes are closely linked to each other. What would be the advantages of GTP-dependent targeting of R-Ras to focal adhesions? First, the coupling of R-Ras activation to targeting would localize the function of R-Ras to a defined region on the plasma membrane, eliminating randomized signalling. Second, a localized high concentration of R-Ras molecules could amplify the signal that mediates cell adhesion. This could lead to the recruitment of other signal molecules and scaffolding proteins, thereby building up the focal adhesion. This is supported by the fact that R-Ras enhances the phosphorylation of focal adhesion kinase (FAK) and p130cas (Kwong et al., 2003). Conversely, deactivation of R-Ras would lead to exclusion of R-Ras from focal adhesions, making it free for a new round of targeting. Deactivation could be mediated by GTPase-activating proteins (GAPs), or through phosphorylation. Interestingly, the effector domain of R-Ras is phosphorylated by an Eph receptor kinase and by the activated Src, leading to suppression of R-Ras-mediated cell adhesion (Zou et al., 1999; Zou et al., 2002). Finally, a specific lipid raft-like composition of the adhesion structure might support protein-protein-based targeting of R-Ras to focal adhesions. The cholesterol content especially may have an important role in modulating the rigidity of focal adhesions (Gopalakrishna et al., 2000), as we show here. Why is H-Ras not localized to focal adhesions? One possibility is that the time during which H-Ras resides in focal adhesions is more transient. This is supported by the fact that H-Ras12V has been found to be associated with focal adhesions at early times of expression and when expressed at relatively low levels (Nobes and Hall, 1999). In addition, activated H-Ras has a more suppressive function on integrins, inducing loss and enhanced turnover of adhesions (Nobes and Hall, 1999; Hughes et al., 1997), which might explain why RRas is excluded from HT1080 cells that possess a mutant Nras allele. Both H-Ras and N-Ras use the endomembrane system to reach the plasma membrane (Choy et al., 1999). We showed that this is also true for R-Ras. Likewise, palmitoylation is crucial for the efficient transport of R-Ras from Golgi onwards. Whether R-Ras proteins are internalized from the plasma membrane is unclear. However, we observed the GDP form of R-Ras on vesicular structures colocalizing with Arf6, suggesting that R-Ras undergoes internalization and recycling (Brown et al., 2001). An interesting possibility is that R-Ras deactivation versus activation is coupled to a membrane-based recycling route that is important for the regulation of adhesion turnover. Future studies in this direction could be important in understanding the functions of focal adhesions. In conclusion, our data on R-Ras further strengthens the importance of specific micro-domains on the plasma membrane as platforms for signalling by small GTPases. 3738 Journal of Cell Science 116 (18) We thank Drs P. Auvinen, P. Lappalainen and Sandra Falck for comments on the manuscript. The Academy of Finland, Helsinki University Foundation, Biocentrum Helsinki, and Nylands Nation supported this research. References Berrier, A. L., Mastrangelo, A. M., Downward, J., Ginsberg, M. and LaFlamme, S. E. (2000). Activated R-ras, Rac1, PI 3-kinase and PKCepsilon can each restore cell spreading inhibited by isolated integrin beta1 cytoplasmic domains. J. Cell Biol. 151, 1549-1560. Brown, F. D., Rozelle, A. L., Yin, H. L., Balla, T. and Donaldson, J. G. (2001). Phosphatidylinositol 4.5-biphosphate and Arf6-regulated membrane traffic. J. Cell Biol. 154, 1007-1117. Chavrier, P., Gorvel, J. P., Stelzer, E., Simons, K., Gruenberg, J. and Zerial, M. (1991). Hypervariable C-terminal domain of rab proteins acts as a targeting signal. Nature 353, 769-772. Choy, E., Chiu, V. K., Silletti, J., Feoktistov, M., Morimoto, T., Michaelson, D., Ivanov, I. E. and Philips, M. R. (1999). Endomembrane trafficking of ras: the CAAX motif targets proteins to the ER and Golgi. Cell 98, 69-80. Cox, A. D., Brtva, T. R., Lowe, D. G. and Der, C. J. (1994). R-Ras induces malignant, but not morphologic, transformation of NIH3T3 cells. Oncogene 9, 3281-3288. Crespo, P. and Leon, J. (2000). Ras proteins in the control of the cell cycle and cell differentiation. Cell Mol. Life Sci. 57, 1613-1636. Gopalakrishna, P., Chaubey, S. K., Manogaran, P. S. and Pande, G. (2000). Modulation of alpha5beta1 integrin functions by the phospholipid and cholesterol contents of cell membranes. J. Cell Biochem. 77, 517-528. Hansen, M., Rusyn, E., Hughes, P., Ginsberg, M., Cox, A. and Willumsen, B. (2002). R-Ras C-terminal sequences are sufficient to confer R-Ras specificity to H-Ras. Oncogene 21, 4448-4461. Hughes, P., Renshaw, M., Pfaff, M., Forsyth, J., Virginia, M., Keivens, V., Schwartz, M. and Ginsberg, M. (1997). Suppression of integrin activation: a novel function of a Ras/Raf-initiated MAP kinase pathway. Cell 88, 521-530. Hughes, P., Oertli, B., Hansen, M., Chou, F.-L., Willumsen, B. and Ginsberg, M. (2002). Suppression of integrin activation by activated Ras or Raf does not correlate with bulk activation of ERK MAP kinase. Mol. Biol. Cell 13, 2256-2265. Kaibuchi, K., Kuroda, S. and Amano, M. (1999). Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu. Rev. Biochem. 68, 459-486. Kwong, L., Wozniak, M., Collins, A., Wilson, S. and Keely, P. (2003). RRas promotes focal adhesion formation through focal adhesion kinase and p130CAS by a novel mechanism that differs from integrins. Mol. Cell. Biol. 23, 933-949. Lee, H., Volonte, D., Galbiati, F., Iyengar, P., Lublin, D. M., Bregman, D. B., Wilson, M. T., Campos-Gonzalez, R., Bouzahzah, B., Pestell, R. G., Scherer, P. E. and Lisanti, M. P. (2000). Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr14) in vivo: identification of a c-Src/Cav-1/Grb7 signaling cassette. Mol. Endocrinol. 14, 1750-1775. Lowe, D. G. and Goeddel, D. V. (1987). Heterologous expression and characterization of human R-ras gene product. Mol. Cell Biol. 7, 2845-2856. Manser, E., Huang, H.-Y., Loo, T.-H., Chen, X.-Q., Dong, J.-M., Leung, T. and Lim, L. (1997). Expression of constitutively active alpha-PAK reveals effects of the kinase on actin and focal complexes. Mol. Cell Biol. 17, 11291143. Manser, E., Leung, T., Salihuddin, H., Zhao, Z.-S. and Lim, L. (1994). A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367, 40-46. Marshall, C. J., Hall, A. and Weiss, R. A. (1982). A transforming gene present in human sarcoma cell lines. Nature 299, 171-173. Michaelson, D., Silletti, J., Murphy, G., D’Eustachio, P., Rush, M. and Philips, M. R. (2001). Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding. J. Cell Biol. 152, 111-126. Nobes, C. D. and Hall, A. (1995). Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53-62. Nobes, C. D. and Hall, A. (1999). Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 144, 1235-1244. Oertli, B., Han, J., Marte, B. M., Sethi, T., Downward, J., Ginsberg, M. and Hughes, P. E. (2000). The effector loop and prenylation site of R-Ras are involved in the regulation of integrin function. Oncogene 19, 4961-4969. Pande, G. (2000). The role of membrane lipids in regulation of integrin functions. Curr. Opin. Cell Biol. 12, 569-574. Parton, R. G. and Hancock, J. F. (2001). Caveolin and Ras function Methods Enzymol. 333, 172-183. Peränen, J. and Furuhjelm, J. (2001). Expression, purification, and properties of Rab8 function in actin cortical skeleton organization and polarized transport. Methods Enzymol. 329, 188-196. Peränen, J., Auvinen, P., Virta, H., Wepf, R. and Simons, K. (1996). Rab8 promotes polarized membrane transport through reorganization of actin and microtubules in fibroblasts. J. Cell Biol. 135, 153-167. Prior, I. A. and Hancock, J. F. (2001). Compartmentalization of Ras proteins. J. Cell Sci. 114, 1603-1608. Prior, I. A., Harding, A., Yan, J., Sluimer, J., Parton, R. G. and Hancock, J. F. (2001). GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nat. Cell Biol. 3, 368-375. Roy, S., Luetterforst, R., Harding, R., Apolloni, A., Etheridge, M., Stang, E., Rolls, B., Hancock, J. F. and Parton, R. G. (1999). Dominant-negative caveolin inhibits H-Ras function by disrupting cholesterol-rich plasma membrane domains. Nat. Cell Biol. 1, 98-105. Sastry, S. K. and Burridge, K. (2000). Focal adhesions: a nexus for intracellular signaling and cytoskeletal dynamics. Exp. Cell Res. 261, 25-36. Schmittberger, T. and Waldmann, H. (1999). Synthesis of the palmitoylated and prenylated C-terminal lipopeptides of the human R- and N-Ras proteins. Bioorg. Med. Chem. 7, 749-762. Self, A. J., Caron, E., Paterson, H. F. and Hall, A. (2001). Analysis of RRas signalling pathways. J. Cell Sci. 114, 1357-1366. Sethi, T., Ginsberg, M. H., Downward, J. and Hughes, P. E. (1999). The small GTP-binding protein R-Ras can influence integrin activation by antagonizing a Ras/Raf-initiated integrin suppression pathway. Biol. Cell 10, 1799-1809. Simons, K. and Toomre, D. (2000). Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1, 31-39. Voice, J. K., Klemke, R. L., Le, A. and Jackson, J. H. (1999). Four human ras homologs differ in their abilities to activate Raf-1, induce transformation, and stimulate cell motility. J. Biol. Chem. 274, 17164-17170. Wang, B., Zou, J. X., Ek-Rylander, B. and Ruoslahti, E. (2000). R-Ras contains a proline-rich site that binds to SH3 domains and is required for integrin activation by R-Ras. J. Biol. Chem. 275, 5222-5227. Wei, Y., Yang, X., Liu, Q., Wilkins, J. A., and Chapman, H. A. (1999). A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J. Cell Biol. 144, 1285-1294. Zhang, Z., Vuori, K., Wang, H., Reed, J. C. and Ruoslahti, E. (1996). Integrin activation by R-ras. Cell 85, 61-69. Zou, J. X., Wang, B., Kalo, M. S., Zisch, A. H., Pasquale, E. B. and Ruoslahti, E. (1999). An Eph receptor regulates integrin activity through R-Ras. Proc. Natl. Acad. Sci. USA 96, 13813-13818. Zou, J. X., Liu, Y., Pasquale, E. B. and Ruoslahti, E. (2002). Activated SRC oncogene phosphorylates R-ras and suppresses integrin activity. J. Biol. Chem. 277, 1824-1827.