* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Electrophoresis Chapter 10 +
Homology modeling wikipedia , lookup
Immunoprecipitation wikipedia , lookup
Protein domain wikipedia , lookup
Protein folding wikipedia , lookup
Protein structure prediction wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Circular dichroism wikipedia , lookup
Protein moonlighting wikipedia , lookup
List of types of proteins wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Protein purification wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
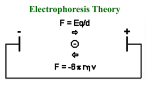
Electrophoresis Chapter 10 ARNE TISELIUS Nobelprize in Chemistry 1948 Motivation: “for his research on electrophoresis and adsorption analysis, especially for his discoveries concerning the complex nature of the serum proteins" Electrophoresis can only be performed on molecules having a charge. + - ++ ++- + ++ - + ++- ++ + - + ++ ++ ++ ++ + + ++ + --- - + 1 Good things with electrophoresis • Relatively simple and inexpensive equipment • High resolution results • Easy to analyze multiple samples • High sensitivity • Specific detection easy (immunological, enzymatic) Different formats • Tubegels: were first used • Vertical slab-gels: most common when separating proteins. • Horizontal slab-gels: IEF and 2D-gels • Capillary electrophoresis 2 Formulas V =E d V = voltage d = distance E = Potential gradient v = Eq f v = velocity q = charge f = friction coefficient v µ= E µ = eletrophoretic mobility Formulas V = I*R W =I2R R = Resistance I = Current V = Voltage W = Effect 3 Heat is generated 1. Increased diffusion -> band broadening 2. Convection currents -> Mixing of sample 3. Heat-sensitive proteins can be damaged. 4. Lower viscosity of the buffer lead so a reduction of the friction coefficient -> faster velocity of the sample. Electroendosmosis - liquid movement (in the capillary) - - - - - - - - - - - - + + + + + + + + - - ++ ++ + ++ - + ++ + ++ + ++ - + ++ ++ ++ ++ + - 4 Supporting media (1) avoid e.g. convection currents Agarose • • • • Polysaccharide (same as in agar) Both intra- and inter-molecular binding 1-3% gels are used Used to separate proteins and DNA. Somewhat large pores for proteins. • Uncharged. However, substitutions may occur (avoid electroendosmosis) • The pores are suitable for size separation of RNA and DNA Supporting media (2) avoid e.g. convection currents Polyacrylamide PAGE (Poly Acrylamide Gel Electrophoresis) • Polymerization of acrylamide and bis-acrylamide, the amount of bis determines the decree of cross-coupling • Catalysis by a free radical: R* +M -> RM* RM* + M -> RMM* etc. 9degas, Oxygen can react with the free radical or create airbubbles in the gel 9Photopolymerization: Riboflavin 9Chemical polymerization : TEMED and ammoniumpersulphate • Depending on the desired frictional constant, 3 to 30% gels are used. 5 SDS-PAGE Sodium-Dodecyl-Sulphate PAGE Separation by size Determination of relative size -> compare with a molecular size marker Control of purity between and after a purification step. Sample: • Boiled with SDS, a detergent CH3-(CH2)10-CH2OSO3-Na+ binding to the protein and denaturating it (1SDS/2 aminosyror) -> all proteins becomes negatively charged, the amount of charge is reflecting the size. • A reducing agent is used to reduce all disulphide bridges. • A color in the sample is reflecting how far the sample has moved, BromphenolBlue (BFB) • Glycerol to increase the density of the sample. Stacking gel The topmost part of the gel where the sample is concentrated to a narrow band • Large pores ( ≈4%) -> low friction, size independent • Not the same buffer, lower pH than the separation gel • Different ion have different mobility: Glycinate< Proteins with SDS <ClMust move with the same velocity – a higher field-strength is needed. This is compensated by a concentration of the sample. [Cl-]>[SDS-proteines]>[glycinate] • When reaching the separation gel, the pH goes up and glycinate increases its mobility and moves faster than the SDS-proteincomplexes. 6 Separation gel The proteins are separated according to size. •The same charge/length results in a mobility which is reflecting the size. • The network in the gel is enhancing this effect, friction. Higher portion of acrylamide for smaller proteins. •The color, BFB, is moving in the front of the sample. •15% gel is separating ≈10-100 kDa Example gel M 7 Native PAGE • Non-denaturating conditions – the activity of the protein is retained. • The proteins are separated according to size (the network of the gel) and charge Is the protein moving in the right direction? • High resolution • Difficult to predict the behavior of the protein. • Staining for activity Gradient gels PAGE • The concentration of acrylamide is varying in the gel, lowest concentration in top of the gel and highest in the bottom. • Is made using a gradient mixer. • Is commonly used in combination with SDS and a stacking gel. • Gives a larger size interval for the analysis. • Proteins of approximately the same size are easier to separate. • The bands get sharper, “pore-size limited” 8 Isoelectric focusing, IEF (1) • Separation by pI • The gel contains a pH-gradient • High resolution, down to 0.01 pH-unit • Often horisontal gels • The sample can be loaded anywhere on the gel. • High voltage (>2500V) leads to generation of heat, cooling is needed. • Micro-heterogeneities in the sample can be analyzed. Isoelectric focusing, IEF (2) • Large pore-size to avoid effects by differences in size of the proteins. Often PAGE, ≈4%, sometimes even agarose for large proteins. • The gel is polymerized as usual but ampholytic molecules are mixed with the acrylamide. A voltage is applied to the gel which leads to a pH gradient. The sample is loaded and separation is performed. When the protein has reached a pH in the gel where its net-charge is =0 it will stop. 9 Example of an IEF-gel 1 2 3 4 5 6 7 5.85 5.20 4.55 4.15 2D-gel electrophoresis (1) combination of IEF (pI) and SDS-PAGE (size) •The first dimension, IEF, is carried out in a rod-shaped gel, d=1-2 mm. •8M Urea and non-ionic detergent. •The gel is incubated with an SDS-containing buffer. •Separation in the second dimension is performed by putting the strip on top of the stacking gel. •High resolution 1000-2000 (10000) proteines can be separated 10 2D-gel electrophoresis (2) Combination of IEF (pI) and SDS-PAGE (size) pI size Proteome analysis • The genome gives an indication of the different proteins that can be expressed in a cell. (≈22000 unique proteins in humans) • The proteome gives an indication of which proteins are expressed in a particular cell-type and to what extent they are expressed at a certain point in time. • Post-translational modifications are important for the function of proteins and can not be deduced with certainty from the DNA-sequence. 11 2-D gels and proteome analysis 1. 2. 3. 4. 5. Separation of proteines by 2dimensional gel elektrophoresis Elution of the proteins from the gel Trypsin digestion Masspectrometry Data-base analysis Or: 1. Elution of the proteins from the gel 2. Mass spectrometry 3. Data-base analysis Staining and analysis of the gel z z z z Most commonly by “Coomassie Brilliant Blue” (0.1% w/v) mixed with Methanol (Ethanol), H2O, Acetic acid. More sensitive is Silver staining, silver-ions are reduced to silver on the protein (Limit of detection is approx 1 ng protein bands) Glycoproteines can be stained with periodic acid-Schiff (PAS) The amount of protein in the bands can be estimated by using a scanning densitometer. It measures the intensity of the bands by scanning with a laser-beam and measuring transmitted light. 12 Western blotting (1) protein blotting Capillary blotting: Capillary forces are used to transfer the proteines to a membrane: Something heavy Dry membrane Nitro-cellulose filter, equilibrated in buffer Gel Electroblotting: A current is used to transfer the proteins to a membrane: + - Nitro-cellulose filter, equilibrated in buffer Gel Western blotting (2) protein blotting ÐBlocking of the nitro-cellulose filter ÐAffinity ligand binding the target protein is applied, usually an antibody ÐSecondary antibody that is labeled is applied, usually with a covalently attached enzyme. ÐA substrate for the enzyme is added. The product should form a colored insoluble precipitate. Enzyme Secondary antibody Primary antibody Target protein 13 Electrophoresis of DNA • Usually an agarose gel, separation by friction, pore-size of the gel. • ´The separated bands can be compared to a marker with DNA pieces of known size. For dsDNA the unit kb (kilobases) is used For ssDNA the unit nt (nucleotides) is used • Electrophoresis is performed totally submerged in buffer (like a submarine). • Glycerol and BFB in the samples • Stacking gel is not needed since the migration through the gel is very slow. • Staining with Ethidium bromide, visible in UV-light • Limit of detection approx 10 ng. DNA sequencing in Polyacrylamide gels • Separation of small ssDNA fragments (up to approx 1000 nt) • DNA sequencing is performed in PAA-gels since fragments differing in size by only one base can be separated. • DNA sequencing was previously performed in slabgels. Now it is usually being performed in cappilaries. 14 Electrophoresis of large DNA molecules • Pulsed-field Electrophoresis, PGFE, is used to separate very large DNA-molecules, 2*103 kb • The angle of the electric field is changed every 60 seconds. Electrophoresis of RNA Northern blotting Adapted from Professor Roger L. Miesfeld The University of Arizona 15