* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 1 Name Chapter 3 Reading Guide Nucleic Acids, Proteins, and
Fatty acid metabolism wikipedia , lookup
Non-coding DNA wikipedia , lookup
DNA supercoil wikipedia , lookup
Transcriptional regulation wikipedia , lookup
Epitranscriptome wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Point mutation wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Western blot wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Gene expression wikipedia , lookup
Restriction enzyme wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Genetic code wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Protein structure prediction wikipedia , lookup
Metalloprotein wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Proteolysis wikipedia , lookup
Deoxyribozyme wikipedia , lookup
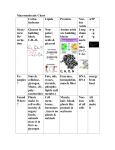
Chapter 3 Reading Guide Nucleic Acids, Proteins, and Enzymes Name _________________________________ Concept 3.1 Nucleic Acids Are Informational Macromolecules 1. What are nucleic acids and what are the two types? 2. What do DNA and RNA function to make proteins. What is the ultimate importance of nucleic acids and the proteins encoded by them? 3. What are the 3 components of a nucleotide? 4. Which of the bases are purines and which are pyrimidines? How do they differ in structure? 5. The structure and function of DNA & RNA the product of base pairing. Which bases pair with each other in DNA? What bases pair in RNA? What type of bonds are the primary bonds between these base pairs? 6. What are the two function of DNA in terms of information? **On page 41 is a list of bullet points about DNA. We will cover this in detail in the future, but do read over it and if you have questions, please feel free to ask. 1 7. If one strand of a DNA molecule has the sequence 5’ – TTCCGGAT – 3’, what is the sequence of the other strand of DNA? If RNA is transcribed from the 5’ - TTCCGGAT – 3’ strand, what would be the sequence? And if RNA is transcribed from the other DNA strand, what would be its sequence? (Note that it is conventional to write these sequences with the 5’ end on the left.) Concept 3.2 Proteins Are Polymers with Important Structural and Metabolic Roles 8. What are the major functions of proteins in living organisms? 9. What are the components of an amino acid? Which portion of the polymer differs in different amino acids? 10. How many animo acids occur extensively in the proteins of all organisms? 11. What are the three major different properties of amino acids that are conferred by their side chains? (You do not have to memorize how many of each type there are.) 2 12. There are 3 “special” amino acids that have side chains that are unique. Briefly describe these 3 amino acids. 13. What type of bonds between amino acids and through what type of reaction do they? 14. What is the difference between oligopeptides and polypeptides? 15. Describe the 4 levels of protein structure. 16. What occurs when a protein is denatured? 17. List and describe some of the factors that can alter protein structure. 3 18. Read the details of the investigation in Fig 3.10 and answer the following questions. a. At what time did disulfide bridges begin to form? b. At what time did enzyme activity begin to appear? c. Explain the difference between your answer for the time of (A) and (B). Disulfide bridges are necessary for protein tertiary structure and must form before the enzyme active site can reappear, but there are other chemical interactions, such as hydrogen bonding and hydrophobic interactions, that occur after the protein has initially folded due to disulfide bridge formation and which are also necessary for enzyme activity. In addition there may be multiple disulfide bridges within the enzyme, all of which must re-form before activity is restored. In other words, the first disulfide bridges to form aren't sufficient to restore activity, so there is a lag before activity reappears. Concept 3.3 Some Proteins Act as Enzymes to Speed up Biochemical Reactions 19. What is the role of a catalyst? 20. What is an enzyme and what is its role? 21. On the graph below, label the axes and the reaction curves to provide a graphic explanation of reactions proceeding with and without enzymes. 4 22. Define the following: a. Activation Energy (Ea)- energy barrier that blocks the tendency for a chemical reaction to occur. b. Transition State – c. Substrate- d. Active Site- 23. What comprises the enzyme-substrate complex and what type of bonds can be responsible for their structure? 24. Label the following diagram to show the action of enzymes. 5 25. After the enzyme – substrate complex is formed, describe the three different mechanisms that enzymes use to catalyze a reaction. 26. What is meant by “induced fit”? 27. What are the three other roles of the enzyme that contributes to the need for them to be large? 28. What is a cofactor? 6 An inorganic ion weakly bound to an enzyme that is required for it to function. 29. Provide an explanation for the following curve showing reactions with and without enzymes. 30. What is a metabolic pathway? How are enzymes key in the regulation of metabolic pathways? Series of enzyme catalyzed reactions in which the product of one reaction is the reactant in the next. 31. The cell can either control the amount or the activity of enzymes in order to control metabolic pathways. Describe the following types of regulation: e. Inhibition – can be irreversible or reversible. A molecule called an inhibitor bonds to the enzyme and renders the enzyme inactive. f. Allosteric Regulation – a non-substrate molecule binds or modifies a site other than the active site. g. Feedback Inhibition – The end product of a reaction may bind with the active site and inhibit it continuing to function. 7