* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Available
Nicotinamide adenine dinucleotide wikipedia , lookup
Photosynthesis wikipedia , lookup
Mitochondrion wikipedia , lookup
Proteolysis wikipedia , lookup
Peptide synthesis wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Genetic code wikipedia , lookup
Microbial metabolism wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Butyric acid wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Nitrogen cycle wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Metalloprotein wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Biochemistry wikipedia , lookup
Amino acid synthesis wikipedia , lookup
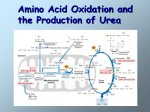
Model Answers B.Sc. (Hons.) (Second Semester) Biotechnology (Biomolecules II) Subject Code: AR-7858 Objective type questions (all compulsory) i. ii. iii. iv. v. vi. vii. viii. ix. x. Formation of glucose from non-carbohydrate source called (b) Gluconeogenesis Sucrose is a (b) Disaccharide How many molecules of ATP are hydrolysed to form two molecule of ammonia? (d) 16 The nitrogen atoms of urea produced in the urea cycle are derived from (a) Ammonia and aspartic acid Which of the following supplies the 2 corbon units that are added to the elongation of fatty acid chain? (b) Malonyl co A Conversion of Acetyl co A to Malonyl co A requires which of the following (a) Biotin FAD is reduced to FADH2 during (c) Krebs cycle A biological redox reaction always involves (a) An oxidized agent, a gain of electron, a reducing agent Which statement best describes Xanthine (b) It is oxidized to form Uric acid Methylated hetrocyclic bases of plants includes all except (b) Thymine Answer-2, TCA Cycle Ans: 3 Nitrogen metabolism in brief: It is the polymeric nitrogen containing compounds proteins and nucleic acids that define the major attributes of organism such as function and structure. Operation and mechanism of metabolic pathways is provided by proteins. Genetic information is stored in nucleic acid polymers. Each of the monomer of these macromolecules has an individual metabolic pathway. In addition, the monomeric nucleotides are essential for energy turnover as key intermediates in all metabolic pathways and also as second messenger molecules, often in form of cyclic nucleotides. Amino acids contribute to carbohydrate synthesis via gluconeogenesis, to fat synthesis or energy production via acetyl-CoA, and special nitrogen compounds such as catecholamines (neurotransmitters), thyroid hormones, creatine(-phosphate), the protoporphyrin ring (heme), and contribute to nucleic acid and phospholipid synthesis as nitrogen group donor. The majority of useful nitrogen for animal metabolism comes from proteins in the form of reusable ammonia (NH3). Nitrogen is fixated in form of ammonia by microorganisms and all 'higher' forms of life (eukaryotes) depend on this primordial source of nitrogen extracted from the air. The 'usefulness' of proteins depends on four distinct properties: 1. total amount of protein ingested 2. digestibility of proteins 3. amino acid composition of proteins 4. total caloric intake Removal of Nitrogen from Amino Acids The dominant reactions involved in removing amino acid nitrogen from the body are known as transaminations. This class of reactions funnels nitrogen from all free amino acids into a small number of compounds; then, either they are oxidatively deaminated, producing ammonia, or their amine groups are converted to urea by the urea cycle. Transaminations involve moving an α-amino group from a donor α-amino acid to the keto carbon of an acceptor α-keto acid. These reversible reactions are catalyzed by a group of intracellular enzymes known as aminotransferases, which generally employ covalently bound pyridoxal phosphate as a cofactor. However, some aminotransferases employ pyruvate as a cofactor. Aminotransferases exist for all amino acids except threonine and lysine. The most common compounds involved as a donor/acceptor pair in transamination reactions are glutamate and αKG, which participate in reactions with many different aminotransferases. Serum aminotransferases such as aspartate aminotransferase, AST (also called serum glutamateoxaloacetate-aminotransferase, SGOT) and alanine transaminase, ALT (also called serum glutamate-pyruvate aminotransferase (SGPT) have been used as clinical markers of tissue damage, with increasing serum levels indicating an increased extent of damage (see the Enzyme Kinetics page for description of the use of enzyme levels in diagnosis). Alanine transaminase has an important function in the delivery of skeletal muscle carbon and nitrogen (in the form of alanine) to the liver. In skeletal muscle, pyruvate is transaminated to alanine, thus affording an additional route of nitrogen transport from muscle to liver. In the liver, alanine transaminase transfers the ammonia to α-KG and regenerates pyruvate. The pyruvate can then be diverted into gluconeogenesis. This process is referred to as the glucose-alanine cycle (see above). The ammonia produced in the latter two reactions is excreted as NH4+ in the urine, where it helps maintain urine pH in the normal range of pH4 to pH8. The extensive production of ammonia by peripheral tissue or hepatic glutamate dehydrogenase is not feasible because of the highly toxic effects of circulating ammonia. Normal serum ammonium concentrations are in the range of 20– 40μM, and an increase in circulating ammonia to about 400μM causes alkalosis and neurotoxicity. A final, therapeutically useful amino acid-related reaction is the amidation of aspartic acid to produce asparagine. The enzyme asparagine synthase catalyzes the ATPrequiring transamidation reaction shown below: Most cells perform this reaction well enough to produce all the asparagine they need. However, some leukemia cells require exogenous asparagine, which they obtain from the plasma. Chemotherapy using the enzyme asparaginase takes advantage of this property of leukemic cells by hydrolyzing serum asparagine to ammonia and aspartic acid, thus depriving the neoplastic cells of the asparagine that is essential for their characteristic rapid growth. In the peroxisomes of mammalian tissues, especially liver, there exists a minor enzymatic pathway for the removal of amino groups from amino acids. L-amino acid oxidase is FMNlinked and has broad specificity for the L-amino acids. A number of substances, including oxygen, can act as electron acceptors from the flavoproteins. If oxygen is the acceptor the product is hydrogen peroxide, which is then rapidly degraded by the catalases found in liver and other tissues. Missing or defective biogenesis of peroxisomes or L-amino acid oxidase causes generalized hyperaminoacidemia and hyperaminoaciduria, generally leading to neurotoxicity and early death. Answer: 4 The Urea Cycle Earlier it was noted that kidney glutaminase was responsible for converting excess glutamine from the liver to urine ammonium. However, about 80% of the excreted nitrogen is in the form of urea which is produced exclusively in the liver, in a series of reactions that are distributed between the mitochondrial matrix and the cytosol. The series of reactions that form urea is known as the Urea Cycle or the Krebs-Henseleit Cycle. Diagram of the urea cycle. The reactions of the urea cycle which occur in the mitochondrion are contained in the red rectangle. All enzymes are in red, CPS-I is carbamoyl phosphate synthetaseI, OTC is ornithine transcarbamoylase. Click on the enzyme names to go to a descriptive page of the urea cycle disorder caused by deficiency in the particular enzyme. The essential features of the urea cycle reactions and their metabolic regulation are as follows: Arginine from the diet or from protein breakdown is cleaved by the cytosolic enzyme arginase, generating urea and ornithine. In subsequent reactions of the urea cycle a new urea residue is built on the ornithine, regenerating arginine and perpetuating the cycle. Ornithine, arising in the cytosol, is transported to the mitochondrial matrix via the action of ornithine translocase encoded by the ORNT1 gene. The ORNT1 transporter is a member of the solute carrier family of transporters and as such is also identified as SLC25A15. In the mitochondria ornithine transcabamoylase catalyzes the condensation of ornithine with carbamoyl phosphate, producing citrulline. The energy for the reaction is provided by the high-energy anhydride of carbamoyl phosphate. Concomitant with ornithine transport into the mitochondria is the export of citrulline, by SLC25A15, to the cytosol where the remaining reactions of the cycle take place. Also important in the function of the urea cycle is the mitochodrial transporter called citrin. Citrin is involved in the mitochondrial uptake of glutamate and export of aspartate and as such functions in the malate-aspartate shuttle. Citrin is a Ca2+-dependent mitochondrial solute transporter that is also a member of the solute carrier family of transporters identified as SLC25A13. The synthesis of citrulline requires a prior activation of carbon and nitrogen as carbamoyl phosphate (CP). The activation step requires 2 equivalents of ATP and the mitochondrial matrix enzyme carbamoyl phosphate synthetase-I (CPS-I). There are two CP synthetases: a mitochondrial enzyme, CPS-I, which forms CP destined for inclusion in the urea cycle, and a cytosolic CP synthetase (CPS-II), which is involved in pyrimidine nucleotide biosynthesis. CPSI is positively regulated by the allosteric effector N-acetylglutamate, while the cytosolic enzyme is acetylglutamate independent. In a 2-step reaction, catalyzed by cytosolic argininosuccinate synthetase, citrulline and aspartate are condensed to form argininosuccinate. The reaction involves the addition of AMP (from ATP) to the amido carbonyl of citrulline, forming an activated intermediate on the enzyme surface (AMP-citrulline), and the subsequent addition of aspartate to form argininosuccinate. Arginine and fumarate are produced from argininosuccinate by the cytosolic enzyme argininosuccinate lyase (also called argininosuccinase). In the final step of the cycle arginase cleaves urea from arginine, regenerating cytosolic ornithine, which can be transported to the mitochondrial matrix for another round of urea synthesis. The fumarate, generated via the action of arginiosuccinate lyase, is reconverted to aspartate for use in the argininosuccinate synthetase reaction. This occurs through the actions of cytosolic versions of the TCA cycle enzymes, fumarase (which yields malate) and malate dehydrogenase (which yields oxaloacetate). The oxaloacetate is then transaminated to aspartate by AST. Beginning and ending with ornithine, the reactions of the cycle consumes 3 equivalents of ATP and a total of 4 high-energy nucleotide phosphates. Urea is the only new compound generated by the cycle; all other intermediates and reactants are recycled. The energy consumed in the production of urea is more than recovered by the release of energy formed during the synthesis of the urea cycle intermediates. Ammonia released during the glutamate dehydrogenase reaction is coupled to the formation of NADH. In addition, when fumarate is converted back to aspartate, the malate dehydrogenase reaction used to convert malate to oxaloacetate generates a mole of NADH. These two moles of NADH, thus, are oxidized in the mitochondria yielding 6 moles of ATP. Answer 5 Essential fatty acids, or EFAs, are fatty acids that humans and other animals must ingest because the body requires them for good health but cannot synthesize them.[1] The term "essential fatty acid" refers to fatty acids required for biological processes but does not include the fats that only act as fuel. Only two fatty acids are known to be essential for humans: alpha-linolenic acid (an omega-3 fatty acid) and linoleic acid (an omega-6 fatty acid).[2][3] Some other fatty acids are sometimes classified as "conditionally essential," meaning that they can become essential under some developmental or disease conditions; examples include docosahexaenoic acid (an omega-3 fatty acid) and gamma-linolenic acid (an omega-6 fatty acid). Answer: 6 A steroid is a type of organic compound that contains a characteristic arrangement of four cycloalkane rings that are joined to each other. Examples of steroids include the dietary fat cholesterol, the sex hormones estradiol and testosterone and the anti-inflammatory drug dexamethasone. The core of steroids is composed of twenty carbon atoms bonded together that take the form of four fused rings: three cyclohexane rings (designated as rings A, B and C in the figure to the right) and one cyclopentane ring (the D ring). The steroids vary by the functional groups attached to this four-ring core and by the oxidation state of the rings. Sterols are special forms of steroids, with a hydroxyl group at position-3 and a skeleton derived from cholestane. Hundreds of distinct steroids are found in plants, animals and fungi. All steroids are made in cells either from the sterols lanosterol (animals and fungi) or from cycloartenol (plants). Both lanosterol and cycloartenol are derived from the cyclization of the triterpene squalene Isoprene, or 2-methyl-1,3-butadiene, is a common organic compound with the formula CH2=C(CH3)CH=CH2. It is a colorless volatile liquid. Isoprene is produced by many plants. Answer: 7 Substrate-level phosphorylation is a type of metabolic reaction that results in the formation of adenosine triphosphate (ATP) or guanosine triphosphate (GTP) by the direct transfer and donation of a phosphoryl (PO3) group to adenosine diphosphate (ADP) or guanosine diphosphate (GDP) from a phosphorylated reactive intermediate. Note that the phosphate group does not have to come directly from the substrate. By convention, the phosphoryl group that is transferred is referred to as a phosphate group. The main part of substrate-level phosphorylation occurs in the cytoplasm of cells as part of glycolysis and in mitochondria as part of the Krebs Cycle under both aerobic and anaerobic conditions. In the payoff phase of glycolysis, two ATP are produced by substrate-level phosphorylation: two and only two 1,3bisphosphoglycerate are converted to 3-phosphoglycerate by transferring a phosphate group to ADP by a kinase; two phosphoenolpyruvate are converted to pyruvate by the transfer of their phosphate groups to ADP by another kinase. 2 molecules of 1,3-bisphoshoglycerate (C3H4O4P2) + phosphoglycerokinase + 2 ADP → 2 molecules of 3phosphoglycerate (C3H5O4P1) + 2 ATP Answer: 8 Pyrimidine Nucleotide Biosynthesis Synthesis of the pyrimidines is less complex than that of the purines, since the base is much simpler. The first completed base is derived from 1 mole of glutamine, one mole of ATP and one mole of CO 2 (which form carbamoyl phosphate) and one mole of aspartate. An additional mole of glutamine and ATP are required in the conversion of UTP to CTP. The pathway of pyrimidine biosynthesis is diagrammed below. The carbamoyl phosphate used for pyrimidine nucleotide synthesis is derived from glutamine and bicarbonate, within the cytosol, as opposed to the urea cycle carbamoyl phosphate derived from ammonia and bicarbonate in the mitochondrion. The urea cycle reaction is catalyzed by carbamoyl phosphate synthetase I (CPS-I) whereas the pyrimidine nucleotide precursor is synthesized by CPS-II. Carbamoyl phosphate is then condensed with aspartate in a reaction catalyzed by the rate limiting enzyme of pyrimidine nucleotide biosynthesis, aspartate transcarbamoylase (ATCase). Enzyme names: 1. aspartate transcarbamoylase, ATCase 2. carbamoyl aspartate dehydratase 3. dihydroorotate dehydrogenase 4. orotate phosphoribosyltransferase 5. orotidine-5'-phosphate carboxylase