* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chara Myosin and the Energy of Cytoplasmic Streaming
Survey
Document related concepts
Tissue engineering wikipedia , lookup
Extracellular matrix wikipedia , lookup
P-type ATPase wikipedia , lookup
Cell growth wikipedia , lookup
Cell culture wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cell membrane wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cellular differentiation wikipedia , lookup
Signal transduction wikipedia , lookup
Endomembrane system wikipedia , lookup
List of types of proteins wikipedia , lookup
Transcript
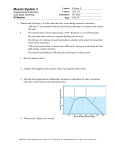
Plant Cell Physiol. 47(10): 1427–1431 (2006) doi:10.1093/pcp/pcl006, available online at www.pcp.oxfordjournals.org ß The Author 2006. Published by Oxford University Press on behalf of Japanese Society of Plant Physiologists. All rights reserved. For permissions, please email: [email protected] Short Communication Chara Myosin and the Energy of Cytoplasmic Streaming Keiichi Yamamoto 1, *, Kiyo Shimada 2, Khoji Ito 1, Saeko Hamada 3, Akio Ishijima 4, Takayoshi Tsuchiya 1 and Masashi Tazawa 5 1 Department of Biology, Chiba University, Inage-ku, Chiba, 263-8522 Japan Chiba Industry Advancement Center, Mihama-ku, Chiba, 261-7126 Japan 3 Department of Life Sciences, Graduate School of Arts and Sciences, The University of Tokyo, Meguro-ku, Tokyo, 153-8902 Japan 4 Institute of Multidisciplinary Research for Advanced Materials, Tohoku University, Aoba-ku, Sendai, 980-8577 Japan 5 Retired from the University of Tokyo in 1990 2 Recently, it was found that myosin generating very fast cytoplasmic streaming in Chara corallina has very high ATPase activity. To estimate the energy consumed by this myosin, its concentration in the internodal cells of C. corallina was determined by quantitative immunoblot. It was found that the concentration of Chara myosin was considerably high (200 nM) and the amount of ATP consumed by this myosin would exceed that supplied by dark respiration if all myosin molecules were fully activated by the interaction with actin. These results and model calculations suggested that the energy required to generate cytoplasmic streaming is very small and only one-hundredth of the existing myosin is enough to maintain the force for the streaming in the Chara cell. Keywords: ATPase — Chara corallina — Cytoplasmic streaming — Maintenance energy — Myosin. Cytoplasmic streaming is unique to and important for plant cells because plant cells, due to their large size, cannot rely on simple diffusion for the transportation and distribution of essential molecules throughout the cell. However, the energy used by plant cells to generate cytoplasmic streaming is not well understood (Bouma 2005). Characean algae have very large cylindrical internodal cells that elongate up to 20 cm with a diameter of 1 mm. Probably owing to this large size, cytoplasmic streaming in characean algal cells is very fast (about 70 mm s1 at 208C). The cytoplasm flows in a direction nearly parallel to the long axis of the cylindrical internodal cell. It goes up along the inner surface of one hemicylinder to the upper node, turns and comes down along the other hemicylinder to the lower node, and then turns and goes up again. It is known that this cytoplasmic streaming is generated by the sliding movement of a myosin (class XI) along the actin cables fixed on the surface of chloroplasts lining the cytosolic face of the cell membrane (Kamiya and Kuroda 1956, Kamitsubo 1966, Nagai and Rebhun 1966, Kachar and Reese 1988, Yamamoto et al. 1994, Kashiyama et al. 2000). Hydrodynamic considerations suggested that movement of myosin alone is not enough to generate the streaming. Myosin should move with its tail attached to the endoplasmic reticulum meshwork to produce bulk flow of water (Nothnagel and Webb 1982). Recently, it was found that the myosin responsible for the streaming in Chara corallina has very high ATPase activity and the activity is 410 times higher than that of skeletal muscle myosin (Ito et al. 2003). The activity of this myosin is so high that, if its concentration in the cell were high, a large amount of ATP would be consumed in the Chara cell by the cytoplasmic streaming. If this is the case, the Chara cell can be a good model to estimate the energy consumed by cytoplasmic streaming in plant cells. We therefore estimated both the amount of this myosin in the Chara cell by quantitative immunoblot and the supply of ATP by metabolic reaction by measuring dark respiration. It was found that the concentration of myosin in the Chara cell was considerably high but the supply of ATP by metabolic reaction was not enough to support the high activity of myosin, if all myosin molecules were fully activated. Since the cytoplasmic streaming of the Chara cell does not stop even at night, we speculate that only a limited amount of myosin interacts with actin cables and generates the force for the cytoplasmic streaming. This idea was supported by the calculations of energy required to generate the cytoplasmic streaming in the Chara cell. We first estimated the amount of myosin in C. corallina internodal cells by quantitative immunoblot as described below. The sum of the length of the internodal cells used was 2,480 mm. We obtained 356 ml of SDS sample from them. A 1 ml aliquot of the sample was subjected to SDS– PAGE and Chara myosin was detected by immunoblot (a typical result is shown in the right end lane of the inserted photo in Fig. 1). When the color development at the myosin band was compared with those of known amounts of globular tail domain (a typical result is shown on the left *Corresponding author: E-mail, [email protected]; Fax, þ81-43-290-2809. 1427 1428 Energy required for cytoplasmic streaming in Chara Color Developement (arbitrary unit) 300 200 100 0 0 1 3 5 7 Applied Tail Domain (ng) Fig. 1 The amount of myosin in the Chara cell estimated by quantitative immunoblot. The standard curve was drawn from the extent of color development of a known amount of globular tail domain expressed molecular biologically (open circle). A typical result of the immunoblot is shown in the inserted photo (left four lanes). The bars indicate the SD of four measurements. Color development at the position of Chara myosin heavy chain was also quantitated (filled circle) under the same conditions. A typical result is also shown in the inserted photo (right end lane). four lanes of the inserted photo in Fig. 1), it corresponded to that of 2.6 ng of globular tail (Fig. 1). It was expected that the signal intensity from the globular tail domain in whole myosin was the same as that from the isolated globular tail domain because they were both blotted onto the membrane after being denatured by SDS. If we consider the difference in the molecular weight between Chara myosin heavy chain (248,000 Da; Kashiyama et al, 2000) and the globular tail domain connected to glutathione S-transferase (86,000 Da) and the transfer efficiency of Chara myosin heavy chain to the nitrocellulose membrane (87%, see below), the amount of Chara myosin in 1 ml was calculated to be 8.6 ng. The total amount of myosin in the SDS sample of internodal cells is, therefore, 3,060 (8.6 356) ng. The total volume of the internodal cells was calculated to be 1,250 ml assuming that the shape of the cell is a cylinder with a radius of 0.4 mm (0.4 0.4 3.14 2,480 mm). Since the volume of the cytoplasm is about 5% of the cell volume, the concentration of Chara myosin heavy chain in cytoplasm was calculated to be 200 nM (0.2 mM). One Chara myosin heavy chain (one active site) hydrolyzes about 1 ATP s1 at 308C and the activity is activated by actin in a concentration-dependent manner. The maximum actin-activated ATPase activity is reported to be 400–500 ATP per heavy chain per second (Ito et al. 2003). If all myosin heavy chains interact with actin and are maximally activated, they consume about 100 mmol ATP s1 l1. The concentration of ATP in the Chara cell is around 5 mM at best and it would be depleted in less than a couple of minutes if a considerable amount of ATP were not supplied by metabolic reaction and photosynthesis. Actually, when we mixed 50 nM recombinant Chara myosin with 4 mM ATP in the presence of 1 mg ml1 F-actin, the ATP decreased in a linear manner to zero in 400 s. This means that the affinity of Chara myosin for ATP is very high and the resultant ADP up to 4 mM does not affect the ATPase activity of Chara myosin. Since cytoplasmic streaming does not stop even at night, it can be said either that the amount of ATP supplied by metabolic reaction is very large in the Chara cell or that only a limited amount of myosin is activated by actin in the cell. To estimate the supply of ATP at night, we measured dark respiration of isolated mature C. corallina internodal cells using an oxygen electrode at 278C. The decrease in oxygen concentration was followed for 42 h in the dark. The rate of decrease was constant during that period, suggesting that the measured value was not a transient one. The value was 0.60 0.15 mg O2 g DW1 h1 (n ¼ 4). Since the ratio of dry weight to fresh weight of Chara cells is 0.077 (T. Tsuchiya and K. Yamamoto unpublished observation), a gram of dry weight corresponds to a cell volume of about 13 ml. If we assume that the P/O ratio is 3 and the volume of cytoplasm is 5% of the total cell volume, ATP supplied by metabolic reaction is 48 mmol s1 l1 at 278C. This value is comparable with those for various characean algae measured by Sorrell et al. (2001) at 158C (11–32 mmol). The result suggests both that ATP supplied by metabolic reaction is not enough to support the fully activated Chara myosin and that not all myosin molecules are working in the cell. The motive force for the cytoplasmic streaming was estimated from the force applied to stop it by using either centrifugation (Kamiya and Kuroda 1958) or vacuolar perfusion (Tazawa 1968, Donaldoson 1972). Kamiya and Kuroda (1973) also measured the velocity gradient in the cytoplasm of a compressed Nitella cell and calculated the motive force. The estimated values were 0.1–0.4 Pa (N m2). The energy required to generate cytoplasmic streaming can be calculated from these values. We imagine an internodal cell having a length and diameter of 10 cm and 0.1 cm (1 mm), respectively. The total inner surface of this cylindrical cell is 3.16 cm2. The total force generated at the interface between the cytoplasm and the surface of chloroplasts where actin cables are fixed is 9.48 105 N (we adopted 0.3 Pa for the motive force). Cytoplasm moves about 100 mm (104 m) in 1 s. The energy required for this movement is 9.48 109 J. When 1 mol of ATP is hydrolyzed to ADP and Pi, the liberated energy is 30.5 kJ. The required ATP is thus 0.311 1012 mol s1. If we assume that the thickness of cytoplasm is 10 mm, Energy required for cytoplasmic streaming in Chara the volume of the cytoplasm is 3.13 106 l. ATP consumed by the cytoplasmic streaming is, therefore, 0.994 101 mmol s1 l1. This value is one- to twothousandth those estimated above assuming that all myosin molecules are fully activated (100 mmol s1) and from dark respiration (48 mmol s1). Because the difference between the estimated values was so great, we tested the validity of the estimated value of the motive force by calculating the mechanical shear force at the interface between the moving cytoplasm and the surface of stationary chloroplasts. It is known that the viscosity of characean cytoplasm is very high so that the cytoplasmic layer moves as a unit in a living cell (Kamiya and Kuroda 1963). We assumed that the Chara cytoplasm is a thin rigid plate with a thickness of 10 mm moving at a velocity of 100 mm s1. The force F required to keep such a plate with the unit area moving at a velocity of v relative to the other parallel plate separated by a distance z can be written as follows; Table 1 1429 Viscosity of bovine serum albumin solution Water Phosphate-buffered saline Bovine serum albumin solution Flow timea Relative densitya Relative viscosity 76.2 79.6 83.0 1.000 1.016 1.024 1.000 1.063 1.115 a Values are the average of three measurements. Moving cytoplasm Chloroplast F ¼ Zdv=dz where Z is the viscosity of a solution filling the space between the two plates. Because the viscosity of Chara cytosol (not cytoplasm) is not known, we estimated it experimentally. We thought that the viscosity of the cytosol was similar to that of a saline solution containing a high concentration of protein and measured the viscosity of 10 mg ml1 bovine serum albumin dissolved in phosphatebuffered saline using an Ostwald viscometer at 208C. The relative density r of solutions was determined by weighing the same volume of solutions sucked with the same liquid dispenser. Flow time t and relative density are shown in Table 1. The viscosity of the albumin solution was calculated as follows: Zsample ¼ Zwater rsample tsample rwater twater The viscosity of water at 208C is 1 103 Pa s. We, therefore, assumed the viscosity of Chara cytosol to be 1.2 103 Pa s. Even if the protein concentration in Chara cytosol were much higher than 10 mg ml1, the viscosity would not exceed 1.5 103 Pa s. We estimated the separation between the cytoplasm and the surface of chloroplast as follows (Fig. 2). There are 3–4 actin cables on one chloroplast and their diameter is 100–200 nm (Kamitsubo 1966, Nagai and Rebhun 1967). The size of Chara myosin observed by electron microscopy is about 100 nm (Yamamoto et al. 1995). Chara cytoplasm contains a meshwork of endoplasmic reticulum membrane and the end of the mesh protrudes about 200 nm from the rest by being pulled by myosin (Kachar and Reese 1988). Cell wall and cell membrane Myosin Cytosol Actin cable Fig. 2 Schematic illustration of the cross-section of Chara internodal cell showing structural elements at the interface between moving cytoplasm and stationary chloroplasts lining the cytosolic face of the cell membrane. The separation thus estimated was 400–500 nm (0.4–0.5 mm). The force calculated from these values was 0.24–0.3 Pa and these values were in good agreement with the values estimated experimentally (Kamiya and Kuroda 1958, Tazawa 1968, Donaldoson 1972, Kamiya and Kuroda 1973). Our calculations suggested that the energy required for the cytoplasmic streaming is very low even in characean algal cells that display very fast cytoplasmic streaming all around the cell. The energy required to generate cytoplasmic streaming was about 0.2% of dark respiration. This is probably the reason why the energy for cytoplasmic streaming has not been considered seriously as a component of the maintenance energy (respiration) in plants. Our results also suggested that myosin was abundant in the Chara cell, but only a limited number of myosin molecules were actively generating force for the cytoplasmic streaming. Because Chara myosin moves very fast, it may not be able to convert the free energy liberated by the hydrolysis of ATP efficiently into mechanical force. Even if we assume that the efficiency is 10%, only one-hundredth of the total myosin is enough to generate the force of cytoplasmic streaming in Chara. Myosin molecules are 1430 Moving cytoplasm A Energy required for cytoplasmic streaming in Chara Tonoplast membrane Endoplasmic reticulum meshwork Chara myosin Actin cable Chloroplast Cell wall and cell membrane B Fig. 3 Localization of myosin in the Chara cell. (a) Schematic illustration showing the localization of Chara myosin. (b) Immunofluorescence localization of Chara myosin. fluorescent spots on actin cables probably represent artifactual myosin stack due to fixation. Glutaraldehyde cannot stop all myosin molecules at once. If one myosin molecule stops moving due to the glutaraldehyde fixation, it will disturb other myosin molecules following the same actin track. This small stack will affect the movement of myosin molecules on the next actin filament in the same cable. An intriguing question is why there are so many myosin molecules in the Chara cell as shown in this study. One reason for this might be the instability of Chara myosin. Chara myosin can move very fast but is, at the same time, very unstable. We have observed that Chara myosin loses its activity very easily during and after the purification process (Yamamoto et al. 1994). Because of this instability, Chara myosin must be recruited frequently from a large store. If this is the case, there must be some regulatory mechanism to suppress unnecessary interaction between Chara myosin and actin to save both ATP and myosin molecules. The other reason might derive from kinetics. Because cytoplasmic streaming in Chara cells is very fast, most of the myosin molecules may fail to grab actin cables. If the probability of this attachment is very low (1–2%) at this streaming velocity, there has to be 50–100 times more myosin molecules than those required for the streaming. A similar decrease in the attachment probability between myosin and actin was suggested to occur in rapidly shortening skeletal muscle (Huxley 1957). Further investigations are required to understand the regulatory mechanism of myosin–actin interaction and the turnover of myosin in Chara. Materials and Methods probably distributed on various kinds of internal membrane but concentrated mostly on the meshwork of endoplasmic reticulum membrane near the actin cables because of its affinity for actin. Active rotation of detached chloroplasts was sometimes observed in the flowing cytoplasm of Chara or Nitella (Kamitsubo and Kikuyama 1992). This can be explained by the interaction between such myosin and a fragment of actin cable attached on the chloroplast surface. Non-specific attachment of Chara myosin to the internal membrane is due to its affinity for acidic phospholipids such as phosphatidylserine (Yamamoto et al. 1995) probably through its globular tail domain. The situation is schematically illustrated in Fig. 3a. Immunofluorescence microscopic observation of Chara cells also revealed that most of the fluorescence antibodies against myosin gathered around actin cables (Fig. 3b). The amount of myosin attached to the other internal membrane may not be so large and we cannot see it clearly due to the strong autofluorescence of chloroplasts. Occasional strongly Expression of the globular tail domain of Chara myosin and its purification was done as described by Awata et al. (2003). Briefly, Gly1639 to Ala2182 of Chara myosin was connected to glutathione S-transferase and the construct was expressed in High Five cells and purified using a glutathione–Sepharose column. The quantitative immunoblot was performed as follows. Antibody against the globular tail domain of Chara myosin was raised in rabbit and purified as described previously (Awata et al. 2003). Crude cytoplasm of C. corallina internodal cells was obtained by squeezing the cell after removing the vacuolar sap by internal perfusion with a solution containing protease inhibitors (Yamamoto et al. 1994). The crude cytoplasm was immediately boiled with SDS and electrophoresed on an 8% polyacrylamide gel. Several known amounts of purified globular tail domain of Chara myosin were also electrophoresed on a separate gel (12%). Proteins were transferred to nitrocellulose membrane under different conditions because of the difference in the transfer efficiency. To examine conditions for the transfer and to estimate the transfer efficiency of Chara myosin heavy chain, we used rabbit skeletal muscle myosin heavy chain because its molecular weight is close to that of Chara myosin and we could easily obtain the myosin in large quantities. Exactly the same amount of muscle Energy required for cytoplasmic streaming in Chara myosin (1 mg) was electrophoresed on the same gel in separate lanes and the gel was cut into two pieces after electrophoresis in between the two lanes. One was stained with Coomassie brilliant blue and the other was used to test the condition for transfer. The latter gel was also stained with Coomassie brilliant blue after the transfer to examine the amount of myosin left in the gel. The amount of myosin transferred to the membrane was examined by Amido Black staining. We used two layers of membrane to examine the amount of myosin passing through the first membrane. The best result was obtained when myosin was electrophoresed in an 8% polyacrylamide gel and transferred to the membrane at 150 mA for 2 h in 25 mM Tris, 192 mM glycine and 0.02% SDS. The transfer efficiency was 87% as judged from the amount of myosin left in the gel before and after transfer, and there was no indication of myosin passing through the first membrane. Similar experiments suggested that electrophoresis in a 12% polyacrylamide gel and transfer to the membrane at 150 mA for 1 h in 25 mM Tris, 192 mM glycine and 0.02% SDS gave the best result for the globular tail domain of Chara myosin. The transfer efficiency was 100%. The membrane was treated with the primary antibody against the globular tail domain of Chara myosin and then the primary antibody was recognized by the anti-rabbit IgG conjugated with alkaline phosphatase. The membrane holding proteins in the Chara cytoplasm and that holding various known amounts of the globular tail domain of Chara myosin were put together in one container and color development by alkaline phosphatase activity was carried out for the same period of time. The color development was quantitated by densitometry and the standard curve was drawn from the values for the globular tail domain. The amount of Chara myosin was determined from the standard curve assuming that the globular tail domain of Chara myosin reacts with the antibody in the same manner as that expressed molecular biologically. The difference in the molecular weight and the transfer efficiency was included in the calculation to estimate the amount of Chara myosin. Dark respiration was measured as follows. Internodal cells of C. corallina were submerged in a 200 ml flask filled with oxygensaturated water in the dark. The ambient water temperature was kept at 278C. The decrease in dissolved oxygen concentration was monitored by an oxygen electrode (UD-101E, Central Science, Tokyo, Japan), furnished through a central hole of the rubber stopper of the flask. The water was vigorously stirred at the bottom by a magnetic stirrer. After the measurement, the internodal cells were oven-dried at 708C for 2 d to measure the dry weight. Respiration was estimated from the rate of decrease in the amount of oxygen dissolved in the flask. Immunofluorescence microscopy was done as follows. Both ends of isolated internodal cells of C. corallina (3–4 cm long) were cut and the vacuole was perfused with a solution containing 80 mM sucrose, 30 mM KCl, 2 mM MgCl2, 2 mM EGTA, 1% (v/v) glutaraldehyde and 4 mM MOPS buffer pH 7.0. Cells were exposed to microwave irradiation using a commercial microwave oven at 500 W for 20 s and left for 60 min at room temperature. Glutaraldehyde was washed with a solution containing 0.2 M sucrose, 70 mM KCl, 5 mM MgCl2, 5 mM EGTA and 10 mM MOPS buffer pH 7.0 (solution A). Cells were perfused with a blocking solution containing 1% gelatin in solution A and left for 10 min. Then cells were treated with antibody against the globular tail domain of Chara myosin dissolved in the blocking solution for 30 min. These cells were washed with solution A and then treated with fluorescein-labeled antibody against rabbit IgG 1431 (Promega, USA) for 30 min. Cells were cut into small segments (1–2 mm in length) and immersed in 50% glycerol containing 0.5% p-phenylenediamine as antifade solution. In this glycerol solution, cylindrical segments were cut to make single layer preparations with the cytosolic face up. The sample was observed and photographed by a Nikon TMD inverted microscope equipped with epifluorescence optics. References Awata, J.Y., Kashiyama, T., Ito, K. and Yamamoto, K. (2003) Some motile properties of fast characean myosin. J. Mol. Biol. 326: 659–663. Bouma,, T.J. (2005) Understanding plant respiration: separating respiratory components versus a process-based approach. Plant respiration. In Advances in Photosynthesis and Respiration, Vol. 18. Edited by Lambers, H. and Ribas-Carbo, M. pp. 177–194. Springer, The Netherlands. Donaldson, I.G. (1972) The estimation of the motive force for protoplasmic streaming in Nitella. Protoplasma 74: 329–344. Huxley, A.F. (1957) Muscle structure and theories of contraction. Prog. Biophys. Biophys. Chem. 7: 255–318. Ito, K., K. Kashiyama, T., Shimada, K., Yamaguchi, A., Awata, J., Hachikubo, Y., Manstein, D.J. and Yamamoto, K. (2003) Recombinant motor domain constructs of Chara corallina myosin display fast motility and high ATPase activity. Biochem. Biophys. Res. Commun. 312: 958–964. Kachar, B. and Reese, T.S. (1988) The mechanism of cytoplasmic streaming in characean algal cells: sliding of endoplasmic reticulum along actin filaments. J. Cell Biol. 106: 1545–1552. Kamitsubo, E. (1966) Motile protoplasmic fibrils in cells of Characeae. II. Linear fibrillar structure and its bearing on protoplasmic streaming. Proc. Jpn Acad. 42: 640–643. Kamitsubo, E. and Kikuyama, M. (1992) Immobilization of endoplasm flowing contiguous to the actin cables upon electrical stimulus in Nitella internodes. Protoplasma 168: 82–86. Kamiya, N. and Kuroda, K. (1956) Velocity distribution of the protoplasmic streaming in Nitella cells. Bot. Mag. Tokyo 69: 544–554. Kamiya, N. and Kuroda, K. (1958) Measurement of motive force of the protoplasmic rotation in Nitella. Protoplasma 50: 144–148. Kamiya, N. and Kuroda, K. (1963) Rotational protoplasmic streaming in Nitella and some physical properties of the endoplasm. Proceedings of the Fourth International Congress on Rheology. Part 4 Symposium on Biorheology, pp. 157–171. Kamiya, N. and Kuroda, K. (1973) Dynamics of cytoplasmic streaming in a plant cell. Biorheology 10: 179–187. Kashiyama, T., Kimura, N., Mimura, T. and Yamamoto, K. (2000) Cloning and characterization of a myosin from characean alga, the fastest motor protein in the world. J. Biochem. 127: 1065–1070. Nagai, R. and Rebhun, L.I. (1966) Cytoplasmic microfilaments in streaming Nitella cells. J. Ultrastruct. Res. 14: 571–589. Nothnagel, E.A. and Webb, W.W. (1982) Hydrodynamic models of viscous coupling between motile myosin and endoplasm in characean algae. J. Cell Biol. 94: 444–454. Sorrel, B.K., Hawes, I., Schwarz, A. and Sutherland, D. (2001) Interspecific differences in photosynthetic carbon uptake, photosynthate partitioning and extracellular organic carbon release by deep-water characean algae. Freshwater Biol. 46: 453–464. Tazawa, M. (1968) Motive force of the cytoplasmic streaming in Nitella. Protoplasma 65: 207–222. Yamamoto, K., Kikuyama, M., Sutoh-Yamamoto, N. and Kamitsubo, E. (1994) Purification of actin based motor protein from Chara corallina. Proc. Jpn Acad. 70: 175–180. Yamamoto, K., Kikuyama, M., Sutoh-Yamamoto, N., Kamitsubo, E. and Katayama, E. (1995) Myosin from alga Chara: unique structure revealed by electron microscopy. J. Mol. Biol. 254: 109–112. (Received May 26, 2006; Accepted September 6, 2006)