* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Anatomic Studies on the Superior Colliculus
Process tracing wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Visual selective attention in dementia wikipedia , lookup
Neuroanatomy wikipedia , lookup
Visual search wikipedia , lookup
Neuroplasticity wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Synaptogenesis wikipedia , lookup
Axon guidance wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Neurostimulation wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
C1 and P1 (neuroscience) wikipedia , lookup
Neuroesthetics wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
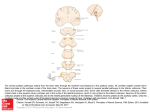
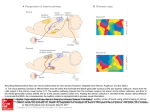
Investigative Ophthalmology June 1972 434 Gouras turtle cones until it was specifically looked for. HUBEL: Isn't it more striking in ganglion cells and couldn't this be accounted for by allowing some of the horizontal cell connections to go on to bipolars as well as on to receptors? GOURAS : I agree, there must be strong opponent interaction after the receptor level but some of this antagonism may also begin in the receptors. Anatomic studies on the superior colliculus R. D. Lund The superior colliculus is organized into a superficial zone which is directly connected with other parts of the visual system and a deep zone which is not directly connected toith other visual centers. Within the superficial zone, there is a convergence of retinal and visual cortical afferents. The size, distribution, and synoptic patterns formed varies between animals. Removal of an eye in neonatal rats, at a time when there are few synapses formed in the colliculus, causes a great increase in the area of distribution of the uncrossed pathway of the remaining eye. At least some of the increased pathway arises from areas of retina which normally would have projected only contralaterally. Removal of an eye at later developmental stages results in no such abnormal projection, although there is evidence that synoptic sites formerly occupied by optic terminals became taken over by other neurons. Key words: superior colliculus, superficial layers, corticotectal pathway, retinotectal pathway, plasticity of connections T_l_he superior colliculi, paired hillocks on the dorsal surface of the midbrain, are concerned with aspects of visual attention and centering of the visual image on the retina and as such are of considerable importance in normal visual function.1"5 Of particular interest is how the processing of visual information through these structures compared with that through the geniculocortical system and to what degree the From the Departments of Biological Structure and Neurological Surgery, University of Washington School of Medicine, Seattle, Wash. 98195. The original work described in this report was supported by United States Public Health Service Grants EY-00491, EY-00596, and NB04053 from the National Institutes of Health. Some facilities were supported by United States Public Health Service Grant HD-02274. Downloaded From: http://iovs.arvojournals.org/ on 06/16/2017 colliculi function independently of the geniculocortical system. Each colliculus is a laminated structure, consisting of seven layers, each defined by the presence or absence of tangentially oriented fiber bundles.0 The layers can be grouped into two major laminar divisions —superficial and deep. The superficial division receives inputs from contralateral and ipsilateral retinas and from visual areas of the ipsilateral cortex and sends fibers to the thalamic nuclei, lateralis posterior and ventral lateral geniculate body,7 and to the deeper laminar division. The deep division, in addition to the input from the superficial layer, receives inputs from somatosensory and auditory systems, from nonvisual cortical areas, and from the contralateral colliculus.810 Its major outputs are to the brainstem, particularly to nuclei involved in coordination of head and eye Volume 11 Number 6 movements,8'a1"13 and there is also an ascending projection to the posterior region of the thalamus.7'8 In this review attention is restricted to the superficial laminar division. It is composed of two tangential fiber layers—the stratum zonale (at the surface) and the stratum opticum (lying deep)—and between them a complex layer of neurons and neuropil—the stratum griseum superficiale. The majority of the visual afferents to the colliculus enter via the stratum opticum and terminate principally in the stratum griseum superficiale. A small number of afferents distribute in the stratum zonale which also contains the axons of intrinsic neurons. It is not known whether the afferents running in this layer are different in function from those entering via the stratum opticum, or whether they are a remnant homologue of the superficial optic layer found in nonmammalian vertebrates.6 The stratum zonale varies in its degree of development between animals, being 10 to 15 fi thick in the monkey,14 an animal in which it is well developed, and completely absent in the ground squirrel.15 A topographic retinal projection can be mapped on the surface of the colliculus such that the more central part of each half visual field lies anteriorly, and the more peripheral part, posteriorly in the contralateral colliculus. However, in some animals (e.g., rat,1(i cat,17 squirrel, tree shrewis) the map continues anteriorly beyond the vertical midline of the visual field so that the most anterior point on the colliculus is about 15 degrees nasal of the midline. Such an overlap does not occur in the retinal projection to the lateral geniculate nucleus.18"20 The topographic map can be determined physiologically or by anatomical degeneration studies after small retinal lesions. In both cases, the optic projection appears as columns tangential to the surface.21- 22 Small lesions of visual cortical areas also cause small patches or columns of degeneration23 in the superficial laminar division of the superior colliculus. The corticotectal map Downloaded From: http://iovs.arvojournals.org/ on 06/16/2017 Superior colliculus 435 is topographically arranged such that an area of cortex receiving from one retinal area projects to an area of the colliculus receiving from the same retinal area.23 There are two interesting problems related to this matching topography, which holds good for rat,23'24 rabbit,25'2G cat,9'27 tree shrew,2S) 29 and monkey.30 In the monkey, the central seven degrees of the retina does not project to the superior colliculus.30 After removal of one eye, no obvious degeneration can be found in the anterior part of the colliculus either by light14-30 or electron microscopy,14 nor can significant autoradiographic label be detected in the same area after injection of the eye with tritiated leucine.31 However, the macular area of the visual cortex does project to this anterior region. The second problem concerns the projection of the corticotectal pathway to the anterior region of the colliculus in the cat. Since the retina temporal to the midline representation projects to the anterior part of the colliculus but not to the lateral geniculate nucleus and hence visual cortex, one might expect on the basis of matching topography that there should be no corticotectal pathway to this region. This is not the case,9 suggesting either that the corticotectal map is less discrete than the retinotectal map or that a special relationship occurs in this region. Despite the correspondence of the retinotectal and corticotectal field maps in a variety of animals, there are some notable differences in the extent of termination of each pathway between animals. Two extremes are shown in Fig. 1. In the rat the retinotectal pathway projects heavily throughout the strata griseum superficiale and zonale, with a higher density of terminals in the more superficial half, while in the monkey retinotectal terminals are restricted to the superficial half of the stratum griseum superficiale.14' 32 In contrast, the corticotectal pathway of the rat terminates sparsely mainly in the deeper half of the stratum griseum superficiale, but in the monkey terminates heavily throughout 436 Lund Investigative Ophthalmology June 1972 35 Vl'H .V. A* I'l't Fig. 1. Diagrammatic cross-section of the left superior colliculus. Details of the rectangle are shown for rat and monkey. In Column 1, fiber patterns are indicated; in Columns 2 and 3, the distribution of crossed and uncrossed optic pathways respectively are shown; and Column 4 shows the distribution of the corticotectal pathway, sz = stratum zonale; sgs = stratum griseum superficiale; so = stratum opticum. the whole depth of the layer. Ground squirrel15 and tree shrew33 both show a pattern similar to the rat, although the corticotectal pathway has a heavier density of termination in the deep half of the stratum griseum superficiale. The retinotectal pathway of the cat resembles that of the rat in its distribution pattern, but the corticotectal pathway more closely resembles that of the monkey, although its density of termination becomes reduced closer to the surface.3'1'35 By the electron microscope, the same general terminal types are recognized as in the lateral geniculate nucleus.30 There are two main differences. First, no obvious encapsulated synaptic zones are seen, although it is apparent in some animals at least that there are zones of complex synaptic formations, albeit poorly delineated from adjacent areas. Second, there are many more clearly identifiable presynaptic dendrites. In rat,32 ground squirrel,15 cat,34' Downloaded From: http://iovs.arvojournals.org/ on 06/16/2017 and monkey,14 the only animals yet studied with the electron microscope, the optic terminals contain spheroidal vesicles and pale mitochondria and are presynaptic to dendrites or dendritic spines, terminals containing flattened vesicles (some of which are presynaptic dendrites), and occasionally to cell bodies. In rat and cat they undergo both primary dense and neurofilamentous reactions in degeneration, while in the monkey the neurofilamentous reaction is very uncommon. The corticotectal pathway in rat synapses only with small dendrites and dendritic spines, but in both cat and monkey it also forms serial synapses. In degeneration it undergoes the primary dense reaction only in rat, ground squirrel, and cat, but in monkey it also undergoes the neurofilamentous reaction. These results indicate that the relative size, distribution, and synaptic relations of retinotectal and corticotectal pathways vary between animals, and on this basis the effect of cortical lesions on tectal function should also vary. This seems to be the case when comparing the collicular physiological response patterns after cortical lesions in ground squirrel37 and rat3S with those of cat.39'40 It is not known whether the variable projections are due to an over-all change in each pathway or to the addition or loss of particular axon populations, in the case of the retinotectal pathway, for example, involving one ganglion cell type. It has been suggested14 that the two major degenerative reactions may differentiate two axon populations in the optic nerve, each of which projects both to the lateral geniculate nucleus and superior colliculus in the rat, and one of which in the monkey projects to the lateral geniculate nucleus and the other to the superior colliculus. In contrast to the crossed optic pathway, which distributes above the stratum opticum, the uncrossed retinotectal pathway terminates within the stratum opticum in many mammals (rat,41 rabbit,42 ground squirrel,15'43 tree shrew), although in cat44 Volume 11 Number 6 and monkey30 it appears to be distributed within the same zone as the crossed pathway. The uncrossed pathway relates to the visual field map in that it is always found anteriorly, but its posterior extent varies between and within animal groups. It is less extensive, for example, in albino than pigmented animals as well as in Siamese compared with regular cats, in which in addition the terminal zone appears restricted to the more posterior part of the colliculus.45 It is of considerable interest to know what factors guide some axons from the retina to join the optic tract of the same side and end in ipsilateral visual centers, while others cross at the chiasma and terminate contralaterally. Studies on Siamese cats indicate that this process may be disturbed, presumably as a result of a genetic defect, such that axons from areas of retina which should have ended in the contralateral lateral geniculate body end instead in the ipsilateral nucleus, although still maintaining the correct topographical distribution.'10 A study we47 undertook on rats has shown that the distribution of the uncrossed pathway can be modified experimentally by removing the opposite eye early in development. In normal albino rats, the uncrossed pathway is extremely small and distributed in the anteromedial region of the stratum opticum (Fig. 2). If one eye is removed at birth, at a time when very few synapses of any kind are found in the colliculus, an uncrossed pathway from the other eye, as charted by degeneration studies at 3 months or more of age, comes to occupy most of the area of the ipsilateral colliculus, terminating mainly in the strata griseum superficiale and zonale. If the first eye is removed from an animal 12 days or older, the uncrossed degeneration due to removal of the other eye at a later time is exactly as in a normal animal. If the first eye is removed at the fifth postnatal day, there is partially increased distribution of the uncrossed pathway of the other eye. A simi- Downloaded From: http://iovs.arvojournals.org/ on 06/16/2017 Superior colliculus 437 Right eye removed at birth Right eye removed at 5 days Right eye removed at I2'days Fig. 2. Diagram of distribution of the left eye of a rat on the superior colliculi when viewed from above. The anterior borders of the colliculi lie uppermost on the page. The solid hatched line indicates the heavy crossed pathway and the fine hatched line indicates the less densely distributed uncrossed pathway. lar pattern of results has been found in the lateral geniculate body.4S There are a number of possible explanations for these results: 1. The apparent degeneration from removal of the second eye is really residual degeneration remaining from removal of the first eye. Control studies show that degeneration can persist for one and onehalf years after a lesion in an adult rat, although for no more than two weeks in a newborn animal. Care has been taken to account for this factor. 2. The increased area of projection may be a sprouting of the regular uncrossed pathway from its normal area of distribution. This is the explanation suggested in previous studies.47'50 It has a major drawback and that is that, by comparison with Amphibia,49 one might expect at birth that the topographic map between retina and tectum is already established and that this 438 Lund map could not be subsequently upset even by experimental lesions. Such sprouting would seem unlikely as a major cause of the increased pathway, since the regular uncrossed pathway is so small compared with the sprouted pathway and the amount of axonal branching required would be enormous. There is no indication of such an involved pattern of branching of degenerating axons, nor is there indication of a major spread of axons from the anteromedial zone to cover the whole colliculus. In addition, the further experiment described below offers evidence of an alternative explanation. 3. Each ganglion cell at birth sends axons to both colliculi, and, as synapses form, one of these atrophies. This proposition cannot be born out by degeneration studies after short-term survivals following unilateral enucleation at birth. 4. Not all the axons have grown past the chiasm at birth. If an eye is removed at birth and the optic nerve degenerates, axons which normally would have crossed the axons in that nerve may not be able to cross them either because of an unbalanced field effect or because they are degenerating. Therefore, they may reroute and end in the ipsilateral tectum instead. There is no clear evidence as to whether all optic nerves have grown past the chiasm at birth, but it is apparent that retinal cell division, possibly involving the ganglion cell layer, continues for a week after birth. One expectation of this hypothesis would be that small lesions in parts of the retina of the intact eye which would normally not be expected to project ipsilaterally should cause ipsilateral degeneration. A subsequent series of experiments has shown this to be the case, both for the superior colliculus and lateral geniculate body.48 5. One final possibility, which would also be compatible with the experiment described above, is that there may after optic degeneration at birth be collateral branching of the remaining optic nerve to supply ipsilateral as well as contralateral colliculi. Downloaded From: http://iovs.arvojournals.org/ on 06/16/2017 Investigative Ophthalmology June 1972 The type of reorganization shown here seems to be in part at least one of redistribution of axons to the side of the brain opposite to that of which they normally project. Another type of reorganization is evident if an eye is removed at any time after synapses are formed and an appropriate minimum survival time allowed (three to five days in animals up to three weeks of age and seven to 14 days in adults).47'50 Under these conditions, there is indication with the electron microscope that the degenerating optic terminals become removed from their postsynaptic sites and replaced by other axon terminals or by neuroglia. The other terminals may contain spheroidal vesicles like the optic terminals, but many contain flattened vesicles. Whether these new connections are functional is not clear. A considerable percentage of them show aggregation of synaptic vesicles along at least part of the membrane. There is reasonable evidence that the presence of synaptic vesicles indicates the potential for transmission at the synapse, and as such allows the possibility for considerable reorganization of functional connections within a deafferented region. Any such reorganization must always be taken into account when assessing the behavioral and physiologic changes caused in response to specific lesions. The author wishes to thank Dr. Jennifer S. Lund for helpful criticism, Mrs. E. Merrick and Mr. R. Cole for technical assistance, and Mrs. C. Lade for valuable secretarial help. REFERENCES 1. Sprague, J. M., and Meikle, T. H.: The role of the superior colliculus in visually guided behavior, Exp. Neurol. 11: 115, 1969. 2. Schneider, G. E.: Contrasting visuomotor functions of tectum and cortex in the golden hamster, Psychol. Forsch. 31: 52, 1967. 3. Schiller, P. H., and Koerner, F.: Discharge characteristics of single units in superior colliculus of the alert rhesus monkey, J. Neurophysiol. 34: 920, 1971. 4. Schiller, P. H.: The role of monkey superior colliculus in eye movement and vision, INVEST. OPHTHALMOL. 11: 451, 1972. Volume 11 Number 6 5. Wurtz, R. H., and Goldberg, M. E.: The primate superior colliculus and the shift of visual attention, INVEST. OPHTHALMOL. 11: 441, 1972. 6. Huber, G. C., and Crosby, E. C.: A comparison of the mammalian and reptilian tecta, J. Comp. Neurol. 78: 133, 1943. 7. Hall, W. C.: Unpublished results, quoted in Sprague, J. M.: The superior colliculus and pretectum in visual behavior, INVEST. OPHTHALMOL. 11: 473, 1972. 8. Altman, J., and Carpenter, M.: Fiber projections of the superior colliculus in the cat, J. Comp. Neurol. 116: 157, 1961. 9. Garey, L. J., Jones, E. G., and Powell, T. P. S.: Interrelationships of striate and extrastriate cortex with the primary relay sites of the visual pathway, J. Neurol. Neurosurg. Psychiatry 31: 135, 1968. 10. Moore, R. Y., and Goldberg, J. M. : Ascending projections of the inferior colliculus in the cat, J. Comp. Neurol. 121: 109, 1963. 11. Nyberg-Hansen, R.: The location and termination of tectospinal fibers in the cat, Exp. Neurol. 9: 212, 1964. 12. Petras, J. M.: Cortical, tectal and tegmental fiber connections in the spinal cord of the cat, Brain Res. 6: 275, 1967. 13. Anderson, M. E., Yoshida, M., and Wilson, V. J.: Influence of superior colliculus on cat neck motoneurons, J. Neurophysiol. 34: 898, 1971. 14. Lund, R. D.: Synaptic patterns in the superficial layers of the superior colliculus of the monkey, Macaca mulatto, Exp. Brain Res. 15: 1972. In press. 15. Lund, R. D.: Unpublished observations. 16. Siminoff, R., Schwassman, H. O., and Kruger, L.: An electrophysiological study of the visual projection to the superior colliculus of the rat, J. Comp. Neurol. 127: 435, 1966. 17. Feldon, S., Feldon, P., and Kruger, L.: Topography of the retinal projection upon the superior colliculus of the cat, Vision Res. 10: 135, 1970. 18. Lane, R. H., Allman, J. M., and Kaas, J. H.: Representation of the visual field in the superior colliculus of the grey squirrel (Sciurus carolinensis) and the tree shrew (Tupaia glis), Brain Res. 26: 277, 1971. 19. Montero, V. M., Brugge, J. F., and Beitel, R. E.: Relation of the visual field to the lateral geniculate body of the albino rat, J. Neurophysiol. 31: 221, 1968. 20. Sanderson, K. J.: The projection of the visual field to the' lateral geniculate and medial interlaminar nuclei in the cat, J. Comp. Neurol. 143: 101, 1971. 21. Mcllwain, J. T., and Buser, P.: Receptive fields of single cells in the cat's superior Downloaded From: http://iovs.arvojournals.org/ on 06/16/2017 Superior colliculus 439 colliculus, Exp. Brain Res. 5: 314, 1968. 22. Lund, J. S., and Lund, R. D.: Unpublished results. 23. Lund, R. D.: Terminal distribution in the superior colliculus of fibers originating in the visual cortex, Nature (Lond.) 204: 1283, 1964. 24. Lund, R. D.: The occipitotectal pathway of the rat, J. Anat. (Lond.) 100: 51, 1966. 25. Giolli, R. A., and Guthrie, M. D.: Organization of projections of visual areas I and II upon the superior colliculus and pretectal nuclei in the rabbit, Brain Res. 6: 388, 1967. 26. Giolli, R. A., and Guthrie, M. D.: Organization of subcortical projections of visual areas I and II in the rabbit. An experimental degeneration study, J. Comp. Neurol. 142: 351, 1971. 27. Garey, L. J.: Interrelationships of the visual cortex and superior colliculus in the cat, Nature (Lond.) 207: 1410, 1965. 28. Abplanalp, P.: Some subcortical connections of the visual system in Tree Shrews and Squirrels, Brain Behav. Evol. 3: 155, 1970. 29. Harting, J. K., and Noback, C. R.: Subcortical projections from the visual cortex in the tree shrew (Tupaia glis), Brain Res. 25: 21, 1971. 30. Wilson, M. E., and Toyne, M. J.: Retinotectal and corticotectal projections in Macaca mulatta, Brain Res. 24: 395, 1970. 31. Hendrickson, A., Wilson, M. E., and Toyne, M. J.: The distribution of optic nerve fibers in Macaca mulatto, Brain Res. 23: 425, 1970. 32. Lund, R. D.: Synaptic patterns of the superficial layers of the superior colliculus of the rat, J. Comp. Neurol. 135: 179, 1969. 33. Abplanalp, P.: The neuroanatomical organization of the visual system in the tree shrew, Folia Primatol. 16: 1, 1971. 34. Sterling, P.: Receptive fields and synaptic organization of the superficial gray layer of the cat superior colliculus, Vision Res. 11: (Suppl. 3) 309, 1971. 35. Lund, R. D.: Structural organization of the superior colliculus and dorsal lateral geniculate body of the rat, J. Physiol. Soc. Jap. 32: 555, 1970. 36. Lund, R. D., and Cunningham, T. J.: Aspects of synaptic and laminar organization of the mammalian lateral geniculate body, INVEST. OPHTHALMOL. 11: 291, 1972. 37. Michael, C. R.: Units in superior colliculus of ground squirrel, J. Gen. Physiol. 10: 2485, 1967. 38. Humphrey, N. K.: Responses to visual stimuli of units in the superior colliculus of rats and monkeys, Exp. Neurol. 20: 312, 1968. 39. Wickelgren, B. G., and Sterling, P.: Influence of visual cortex on receptive fields 440 Lund 40. 41. 42. 43. 44. 45. 46. 47. 48. 49. 50. in the superior colliculus of the cat, J. Neurophysiol. 32: 1, 1969. Rosenquist, A. C , and Palmer, L. A.: Responses of single cells in the cat superior colliculus after cortical lesions, Anat. Rec. 169: 414, 1971. Lund, R. D.: Uncrossed visual pathways of hooded and albino rats, Science 149: 1506, 1965. Giolli, R. A., and Guthrie, M. D.: The primary optic projections in the rabbit. An experimental degeneration study, J. Comp. Neurol. 136: 99, 1969. Tigges, J.: Retinal projections to subcortical optic nuclei in diurnal and nocturnal squirrels, Brain Behav. Evol. 3: 121, 1970. Laties, A. M., and Sprague, J. M.: The projection of optic fibers to the visual centers in the cat, J. Comp. Neurol. 127: 35, 1966. Kalil, R. E., Jhaveri, S. R., and Richards, W.: Anomalous retinal pathways in the Siamese cat: An inadequate substrate for normal binocular vision, Science 174: 302, 1971. Guillery, R. W., and Kaas, J. H.: A study of normal and congenitally abnormal retinogeniculate projections in cats, J. Comp. Neurol. 143: 73, 1971. Lund, R. D., and Lund, J. S.: Synaptic adjustment after deafferentation of the superior colliculus of the rat, Science 171: 804, 1971. Lund, R. D., Cunningham, T. J., and Lund, J. S.: Unpublished observations. Jacobson, M.: Development of neuronal specificity in retinal ganglion cells of Xenopus, Develop. Biol. 17: 202, 1968. Lund, R. D., and Lund, J. S.: Modification of synaptic patterns in the superior colliculus of the rat during development and following deafferentation, Vision Res. ll:(Suppl. 3) 281, 1971. Discussion SPRAGUE: IS the synaptic takeover you described age dependent? Is it specific for S or F type terminals or is it nonspecific? LUND: Both S or F type terminals are involved in the takeover. It appears nonspecific within the limits of our technique, but this may only say that our technique is nonspecific. We have found the takeover in all animals in which there are formed synapses at the time of the lesion. In animals lesioned at the time when there is a massive formation of optic synapses (15 to 25 days postnatal), there are always a higher percentage of abnormal synapses than at subsequent survival times. BURKE: IS there competition between nerve and glial cells in this? LUND: It appears that both neuronal and neu- Downloaded From: http://iovs.arvojournals.org/ on 06/16/2017 Investigative Ophthalmology June 1972 roglial processes (astrocytes and oligodendrocytes) can come to occupy postsynaptic sites as they become vacated by degenerating optic terminals. Whether they compete and the process is nonspecific (perhaps just to block off hyperactive receptor sites) or whether certain neuronal processes may subsequently come to occupy sites initially covered by glial processes is unclear. So far we have defined the system. The next step would appear, to be to investigate this problem of specificity both between neurons and between neurons and neuroglia. WURTZ: Is there any Golgi material in monkey comparable to what you showed in rat? LUND: T. Langer and I have spent some time looking at Golgi preparations of the monkey colliculus. All the cell types seen in the rat can be found in the monkey. There are a number of differences: (1) There is always a preponderance of pyriform cells staining (these are also shown by Sterling in the cat [Vision Res. 11: Suppl. 3]). (2) The clear lamination in rat of upper and lower stratum griseum superficiale is not obvious. (3) The horizontal cells impregnated so far do not have long processes as have been found in the rat. (4) There are some cells with dendrites which pass through the stratum opticum into the stratum griseum intermedium. This means that units recorded in the stratum griseum superficiale may be activated directly by pathways terminating in the deeper layers. HUBEL: In the cat it is said that there is about 20 degrees or so of ipsilateral field projection which is a puzzle, since it's hard to think of it coming from 17, 18, or 19. Presumably this may come via the optic nerve but then you'd wonder whether the receptive fields would be the same. Is there any evidence for an ipsilateral projection in the monkey? LUND: Neither Schiller and Koerner (J. Neurophysiol. 34: 920, 1971) nor Kadoya et al. (J. Comp. Neurol. 142: 495, 1971) found units in the rostral part of monkey colliculi crossing the midline to any degree, as they do in cat and some other animals. MARK: Have you considered the possibility of axonal sprouting at the chiasm? LUND: This is, of course, one possibility which must be considered. It could perhaps be eliminated by firing optic terminals in the ipsilateral colliculus antidromically and seeing whether a response could be recorded in the other colliculus. SCHILLER: One of my greatest puzzles with respect to the colliculus has to do with retinal versus cortical contributions to cell function. I am particularly puzzled in monkey with the reports you just gave as well as those of Wilson and Toyne. We have found in recording from monkeys after ablations of visual cortex that the receptive field characteristics in these superficial layers do not Superior colliculus 441 Volume 11 Number 6 seem to be affected very dramatically and can subserve foveal vision as well. Have you any ideas on what this cortical input is doing as well as on the anatomy of the lower layers of the colliculus about which you haven't talked but which may clarify some things? LUND: That really is puzzling. I think it highly unlikely that there is a major direct optic projection to the rostral colliculus. It cannot be demonstrated by light or electron microscope degenerative methods, nor can it be shown by autoradiographic labeling methods. Assuming that your cortical lesions are complete and that your electrodes really are in the superior colliculus and not in adjacent .pretectal nuclei, one has to postulate that there may be indirect optic influences via possible pretectum-collicular or geniculate-collicular pathways. Alternatively, there could be a very small number of direct optic terminals to the region which are below the resolution of detection by the anatomical methods used but which, are sufficient to produce unit activity. Your comments regarding the relatively normal response patterns in the colliculus after cortical lesions suggest to me two possibilities—either your experimental situation does not exploit the normal activity parameters of the corticotectal pathway, or the widely assumed anatomical dogma that the size of a pathway bears a direct relation to its magnitude of influence is mistaken. With regard to the last, I think it would be very valuable to test the effects of cortical lesions in monkeys and rats (or ground squirrels) under absolutely identical experimental conditions. As is described here, these animals show marked differences in the relative sizes of the two pathways (retinotectal and corticotectal) and one might expect that this should be reflected in functional differences. The deep layers with the possible exception of part of the stratum griseum intermedium do not receive a projection from visual cortical areas. This does not, of course, mean that they do not respond to more indirect visual influences. The.primate superior colliculus and the shift of visual attention Robert H. Wurtz and Michael E. Goldberg .he primate oculomotor system can generate eye movements in response to visual stimuli. The superior colliculus, long thought to be a component of that system, occupies a transitional point in the oculomotor system. It receives input directly from the retina and indirectly from the visual cortex,9-22 and many investigators have demonstrated cells with visual receptive fields in the superior colliculus of monkeys and other mammals.17 In the primate, the anatomic connections to the motor nuclei of the extraocular muscles are not direct.11 However, stimulating the colliculus results in eye movements14 and single cells in the From the Laboratory of Neurobiology, National Institute of Mental Health, Bethesda, Md. Downloaded From: http://iovs.arvojournals.org/ on 06/16/2017 colliculus discharge before voluntary eye movements.15'20> 23 Although it is clear that the superior colliculus is in the pathway between the visual stimulus and the eye movement, the role played by the colliculus in the process of generating a visually guided eye movement is not at all clear. One view of colliculus function is that it provides the target information necessary for the accurate guidance of eye movements.13"15 However, two different lines of evidence are highly dissonant with this view. The first is that monkeys without superior colliculi show no gross deficit in visually guided eye movements.1' 12 The second is that the functional properties of the cells in the monkey superior colliculus, which have large receptive fields or respond before eye move-