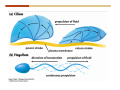
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 1. Describe the steps of the scientific method. 2. Define the terms
Lipid bilayer wikipedia , lookup
Cytokinesis wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell encapsulation wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
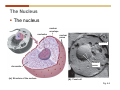
Cell nucleus wikipedia , lookup
Signal transduction wikipedia , lookup
Cell membrane wikipedia , lookup
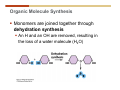
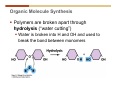
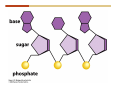
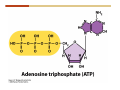
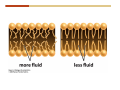
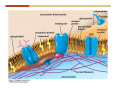
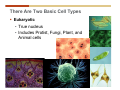
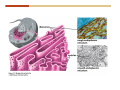
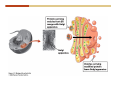
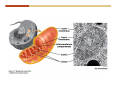
1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. Describe the steps of the scientific method. Define the terms hypothesis, theory, and law. What are the common characteristics of all living things? Describe the levels of organization of life beginning with the smallest living unit and progressing up in complexity to ecosystems. Compare and contrast DNA and RNA. Describe the 3 types of molecular bonds. Which is strongest and which is weakest? What properties of water make it important for life? Briefly describe each of these properties. What properties of Carbon make it important for life? How do those properties make life possible? How are biological molecules formed? How are they broken down? What are carbohydrates and what functions do they perform? What are proteins and what functions do they perform? What are lipids and what functions do they perform? What are nucleic acids and what functions do they perform? What is a plasma membrane? What is it composed of? What functions does it serve? Define diffusion and osmosis. Describe the processes of passive transport across a plasma membrane. Describe the processes of active transport across a plasma membrane. Compare and contrast prokaryotic and eukaryotic cells. Describe the form and function of the organelles common to most eukaryotic cells. Describe the endosymbiont hypothesis of organelle formation and provide an example. Scientific Principles Biology is a scientific discipline All scientific inquiry is based on a small set of assumptions or principles • Natural causality – events have natural causes • Uniformity in space and time • Similar perception – observations of other humans are reliable The Scientific Method Scientific inquiry is a rigorous method for making observations The Scientific Method for inquiry follows stepwise… The Scientific Method Scientific experimentation tests the assertion that a single variable causes a particular observation The experiment must rule out the influence of other possible variables on the recorded observations Controls are incorporated into experiments Controls keep untested variables constant Scientific method is illustrated by Francesco Redi’s experiment Limitations of the Scientific Method Can never be sure all untested variables are controlled Conclusions based on the experimental data must remain tentative Results of experimentation must be communicated thoroughly and accurately to other scientists for repetition Repetition by other scientists adds verification that findings can be used as the basis for further studies Scientific Theory A scientific theory is a general explanation for important natural phenomena • It is extensively and reproducibly tested • It is more like a principle or natural law (e.g. atomic, gravitational, and cell theories) • If compelling evidence arises, a theory may be modified Biosphere Ecosystem • All organisms • All abiotic factors Community Population Organism • • • • • organ systems organs tissues cells molecules ECOSYSTEM LEVEL Eucalyptus forest COMMUNITY LEVEL All organisms in eucalyptus forest POPULATION LEVEL Group of flying foxes ORGANISM LEVEL Flying fox Brain ORGAN SYSTEM LEVEL Nervous system ORGAN LEVEL Brain Spinal cord Nerve TISSUE LEVEL Nervous tissue CELLULAR LEVEL Nerve cell MOLECULAR LEVEL Molecule of DNA Figure 1.1 1.3 What Is Life? Characteristics of living things Living things are organized and complex. Living things grow and reproduce. Living things respond to stimuli. Living things acquire and use material and energy. • Living things use DNA to store information. • • • • Fig. 1-8 Each level of organization builds on the one below it At each level, new properties emerge Biological function starts at the chemical level ATOMS AND MOLECULES 2.1 What Are Atoms? Elements: substances that cannot be broken down by ordinary chemical means (ex/ carbon) all atoms belong to one of 96 types of naturally occurring elements life requires about 25 of these elements 2.1 What Are Atoms? Atoms: basic structural unit of matter consist of charged particles protons (+) neutrons (0) electrons (-) smallest particle of an element each element has a unique number of protons (atomic number) Atoms are electrically neutral because they have and equal number of positive protons and negative electrons 2 Protons Nucleus 2 Neutrons 2 Electrons Helium atom Electrons are arranged in shells Electrons orbit around atomic nuclei at specific distances called electron shells the outermost shell determines the chemical properties of an atom Outermost electron shell (can hold 8 electrons) Electron HYDROGEN (H) Atomic number = 1 First electron shell (can hold 2 electrons) CARBON (C) Atomic number = 6 NITROGEN (N) Atomic number = 7 OXYGEN (O) Atomic number = 8 Energy Capture and Release Life depends on electrons capturing and releasing energy • Electron shells correspond to energy levels • Energy exciting an atom causes an electron jump from a lower- to higher-energy shell • Later, the electron falls back into its original shell, releasing the energy 2.2 How Do Atoms Form Molecules? Molecules: two or more atoms of one or more elements held together by interactions among their outermost electron shells • Atoms interact with one another according to two basic principles: • An inert atom will not react with other atoms when its outermost electron shell is completely full or empty. • A reactive atom will react with other atoms when its outermost electron shell is only partially full. Atoms Interact Reactive atoms gain stability by electron interactions (chemical reactions) • Electrons can be lost to empty the outermost shell • Electrons can be gained to fill the outermost shell • Electrons can be shared with another atom where both atoms have full outermost shells • When atoms combine to fill their outer shells they gain stability Ionic Bonds Formed by passing an electron from one atom to another One partner becomes positive, the other negative, and they attract one another. • Na+ + Cl– becomes NaCl (sodium chloride) Positively or negatively charged atoms are called ions. • + cation • - anion Covalent Bonds Atoms with partially full outer electron shells can share electrons Two electrons (one from each atom) are shared in a single covalent bond Covalent bonds are found in H2 (single bond), O2 (double bond), N2 (triple bond) and H2O Covalent bonds are much stronger than ionic bonds but vary in their stability Covalent Bonds Covalent bonds produce either nonpolar or polar molecules. Nonpolar molecule: atoms in a molecule equally share electrons that spend equal time around each atom, producing a nonpolar covalent bond Polar Covalent Bonds Atoms within a molecule may have different nuclear charges Those atoms with greater positive nuclear charge pull more strongly on electrons in a covalent bond A molecule with polar bonds may be polar overall H2O is a polar molecule • The (slightly) positively charged pole is around each hydrogen • The (slightly) negatively charged pole is around the oxygen Hydrogen Bonds Polar molecules like water have partially charged atoms at their ends Hydrogen bonds form when partial opposite charges in different molecules attract each other The partially positive hydrogens of one water molecule are attracted to the partially negative oxygen on another Hydrogen bonds are rather weak but can collectively be quite strong Hydrogen bonds H (+) O (–) H (+) H (+) O (–) H (+) hydrogen bonds Fig. 2-7 Why Is Water So Important To Life? Water interacts with many other molecules. • Oxygen released by plants during photosynthesis comes from water. • Water is used by animals to digest food. • Water is produced in chemical reactions that produce proteins, fats, and sugars. Why Is Water So Important To Life? Many molecules dissolve easily in water. • Water is an excellent solvent, capable of dissolving a wide range of substances because of its positive and negative poles. • example NaCl dropped into H2O • The positive end of H2O is attracted to Cl–. • The negative end of H2O is attracted to Na+. • These attractions tend to pull apart the components of the original salt. Why Is Water So Important To Life? Water-insoluble molecules are hydrophobic • Water molecules repel and drive together uncharged and nonpolar molecules like fats and oils • The “clumping” of nonpolar molecules is called hydrophobic interaction Why Is Water So Important To Life? Hydrogen bonding between water molecules causes them to stick together. • Cohesion: water molecules stick together • Water molecules can form a chain in delivering moisture to the top of a tree • Cohesion of water molecules along a surface produces surface tension Water molecules stick to polar or charged surfaces in the property called adhesion • Adhesion helps water climb up the thin tubes of plants to the leaves (capillary effect) Why Is Water So Important To Life? Water can form ions. • Water dissociates to become H+ and OH–. • The relative abundance of ions determine pH (–) O H water (H2O) O H (+) + H H hydroxide ion (OH–) hydrogen ion (H+) Acid, Basic, and Neutral Solutions The relative concentrations of H+ and OHions determine pH. • Acid solutions have more H+ (protons). • Alkaline solutions have more OH– (hydroxyl ions). • A base is a substance that combines with H+, reducing their numbers. • pH measures the relative amount of H+ and OH– in a solution. Acid, Basic, and Neutral Solutions The degree of acidity of a solution is measured using the pH scale • pHs 0-6 are acidic (H+ > OH-) • pH 7 is neutral (H+ = OH-) • pH 8-14 is basic (OH- > H+) Acid, Basic, and Neutral Solutions A buffer is a compound that accepts or releases H+ in response to pH change The bicarbonate buffer found in our bloodstream prevents pH change Why Is Water So Important To Life? Water stabilizes temperature • Temperature reflects the speed of molecular motion • It requires 1 calorie of energy to raise the temperature of 1g of water 1oC (specific heat), so it heats up very slowly • Because it heats up very slowly water moderates the effect of temperature change • Very low or very high temperatures may damage enzymes or slow down or halt important chemical reactions Water Stabilizes Temperature Water requires a lot of energy to turn from liquid into a gas (heat of vaporization) • Evaporating water uses up heat from its surroundings, cooling the nearby environment (ex/ sweating) Water requires a lot of energy to be withdrawn in order to freeze (heat of fusion) • Therefore the nearby environment will be warmer than it otherwise would be Water Forms an Unusual Solid: Ice Most substances become more dense when they solidify from a liquid Water molecules spread slightly during crystallization (freezing) Because of this ice is less dense than liquid water Water Forms an Unusual Solid: Ice Because of its lower density ice floats in liquid water Ponds and lakes freeze from the top down Lower water is protected by the surface layer of ice. • Large bodies of water rarely freeze completely • Life can survive in cold water underneath ice. • Spring thaw pushes nutrient-rich bottom water to surface Why Is Water So Important To Life? Like no other common substance on earth, water naturally exists in all three physical states: solid, liquid, and gas Organic vs. Inorganic in Chemistry Organic refers to molecules containing a carbon skeleton Inorganic refers to carbon dioxide and all molecules without carbon 2.4 Why Is Carbon So Important To Life? Carbon can combine with other atoms in many ways to form a huge number of different molecules. Carbon has four electrons in its outermost shell, leaving room for four more electrons from other atoms (4 covalent bonds). Carbon atoms are versatile and can form single, double, or triple bonds and rings. Why Is Carbon So Important To Life? Arrangement of atoms determines molecular shape. Shape determines function of molecules Structural formula Ball-and-stick model Space-filling model Methane The 4 single bonds of carbon point to the corners of a tetrahedron. Why Is Carbon So Important To Life? The great variety of substances found in nature is constructed from a limited pool of atoms. Organic molecules have a carbon skeleton and some hydrogen atoms. Much of the diversity of organic molecules is due to the presence of functional groups. Functional (R) Groups Functional groups in organic molecules confer chemical reactivity and other characteristics • groups of atoms that participate in chemical reactions • determine the chemical properties of molecules • Examples: acidity, solubility What affects solubility in water? Molecules with +/- charge are usually hydrophilic or “water-loving” Molecules with no charge and non-polar are usually hydrophobic and not soluble in water 2.5 How Are Biological Molecules Joined Together Or Broken Apart? Biomolecules are polymers (chains) of subunits called monomers A huge number of different polymers can be made from a small number of monomers Biomolecules Are Joined Through Dehydration and Broken by Hydrolysis Organic Molecule Synthesis Monomers are joined together through dehydration synthesis An H and an OH are removed, resulting in the loss of a water molecule (H2O) Organic Molecule Synthesis Polymers are broken apart through hydrolysis (“water cutting”) Water is broken into H and OH and used to break the bond between monomers Organic Molecule Synthesis All biological molecules fall into one of four broad categories: • • • • Carbohydrates Lipids Proteins Nucleic Acids 2.6 What Are Carbohydrates? Composition: C, H, and O in the ratio of 1:2:1 Types by size: • • • Simple or single sugars are monosaccharides Two linked monosaccharides are disaccharides Long chains of monosaccharides are polysaccharides Monosaccharides Basic monosaccharide structure • • • Backbone of 3-7 carbon atoms Many –OH and –H functional groups Usually found in a ring form in cells Simple sugars provide important energy sources for organisms. Most small carbohydrates are watersoluble due to the polar OH functional groups Disaccharides Disaccharides are two-part sugars • Sucrose (table sugar) = glucose + fructose • Lactose (milk sugar) = glucose + galactose • Maltose (malt sugar)= glucose + glucose glucose fructose CH2OH O H H HOCH2 CH2OH O O H H + HO OH H H OH OH sucrose HO H OH HO H dehydration CH2OH synthesis H H HO HOCH2 H OH H H OH O H OH H O H O H HO H CH2OH Polysaccharides Monosaccharides are linked together to form chains (polysaccharides) Polysaccharides are used for energy storage and structural components Polysaccharides Storage polysaccharides • Starch (polymer of glucose) • Formed in roots and seeds as a form of glucose storage • Glycogen (polymer of glucose) • Found in liver and muscles Polysaccharides Structural polysaccharides • Cellulose (polymer of glucose) • Found in the cell walls of plants • Indigestible for most animals due to orientation of bonds between glucoses • Chitin (polymer of modified glucose units) • Found in the outer coverings of insects, crabs, and spiders • Found in the cell walls of many fungi 2.7 What Are Lipids? Molecular characteristics of lipids • Lipids are molecules with long regions composed almost entirely of carbon and hydrogen. • The nonpolar regions of carbon and hydrogen bonds make lipids hydrophobic and insoluble in water. What Are Lipids? Lipids are diverse in structure and serve in a variety of functions • • • • Energy storage Waterproofing Membranes in cells Hormones What Are Lipids? Lipid classification • Group 1: Oils, fats, and waxes • Group 2: Phospholipids • Group 3: Steroids Lipids Group 1: Oils, fats, and waxes • Formed by dehydration synthesis • 3 fatty acids + glycerol triglyceride • Contain only carbon, hydrogen, and oxygen • Contain one or more fatty acid subunits in long chains of C and H with a carboxyl group (–COOH) • Ring structure is rare Lipids Group 1: Oils, fats, and waxes (continued) • Fats and oils form by dehydration synthesis from three fatty acid subunits and one molecule of glycerol. etc. CH2 CH2 CH2 H H C OH O CH HO C CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH H C OH O HO C CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 etc. H C OH H glycerol + O HO C CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 etc. fatty acids Fig. 2-16 Lipids Group 1: Oils, fats, and waxes (continued) • Fats and oils formed by dehydration synthesis are called triglycerides. • Triglycerides are used for long-term energy storage in both plants and animals. etc. CH2 CH2 CH2 H O CH H C O C CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH + O H O O H C O C CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 etc. + H O H C O C CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 etc. H + H triglyceride H H O H 3 water molecules Fig. 2-16 Lipids Group 1: Oils, fats, and waxes (continued) • Characteristics of fats • Solidity is due to the prevalence of single or double carbon bonds • Fats are solid at room temperature. • Fats have all carbons joined by single covalent bonds. • The remaining bond positions on the carbons are occupied by hydrogen atoms. Lipids Group 1: Oils, fats, and waxes (continued) • Fatty acids of fats are said to be saturated and are straight molecules that can be stacked. (a) Beef fat (saturated) Fig. 2-18a Lipids Group 1: Oils, fats, and waxes (continued) • Characteristics of oils • Oils are liquid at room temperature. • Some of the carbons in fatty acids have double covalent bonds. • There are fewer attached hydrogen atoms, and the fatty acid is said to be unsaturated. Lipids Group 1: Oils, fats, and waxes (continued) • Unsaturated fatty acids have bends and kinks in fatty acid chains and can’t be efficiently stacked. (b) Peanut oil (unsaturated) Fig. 2-18b Lipids Group 1: Oils, fats, and waxes (continued) • Characteristics of waxes • Waxes are solid at room temperature. • Waxes are highly saturated. • Waxes are not a food source. • Waxes are composed of long hydrocarbon chains and are strongly hydrophobic Lipids Group 1: Oils, fats, and waxes (continued) • Waxes form waterproof coatings • Leaves and stems of plants • Fur in mammals • Insect exoskeletons • Used to build honeycomb structures Lipids Group 2: Phospholipids • Phospholipids: form dual layered plasma membranes around all cells • Construction • like oils except one fatty acid is replaced by a phosphate group attached to glycerol. • 2 fatty acids + glycerol + a short polar functional group • water-soluble heads and water-insoluble tails. Lipids Group 2: Phospholipids (continued) • The phosphate end of the molecule is water soluble; the fatty acid end of the molecule is water insoluble. CH3 O– H3C - N+- CH2 - CH2 -O-P- O -CH2 O CH3 O HC-O-C- CH2 -CH2 - CH2 - CH2 - CH2 - CH2 - CH2 -CH O CH CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH3 H2C-O-C- CH2 -CH2 - CH2 - CH2 - CH2 - CH2 - CH2 - CH2 - CH2 - CH2 - CH2 - CH2 - CH2 - CH2 - CH2 -CH3 polar head (hydrophilic) glycerol fatty acid tails (hydrophobic) Fig. 2-19 Lipids Group 3: Steroids • Steroids contain four fused carbon rings. • Various functional groups protrude from the basic steroid “skeleton”. • Examples of steroids • Cholesterol • Found in membranes of animal cells • Male and female sex hormones 2.8 What Are Proteins? Functions of proteins • Proteins act as enzymes to catalyze (speed) many biochemical reactions. • They provide structure (ex/ elastin) • They can act as energy stores. • They are involved in carrying oxygen around the body (hemoglobin). • They are involved in muscle movement. Proteins are formed from chains of amino acids. All amino acids have the same basic structure: • • • • A central carbon An attached amino group An attached carboxyl group An attached variable group (R variable group) group • Some are hydrophobic • Some are hydrophilic amino group hydrogen carboxylic acid group Amino acid monomers join to form chains by dehydration synthesis. • Proteins are formed by dehydration reactions between individual amino acids. • The –NH2 group of one amino acid is joined to the –COOH group of another, with the release of H2O and the formation of a new peptide (two or more amino acids). • The resultant covalent bond is a peptide bond Long chains of amino acids are known as polypeptides or just proteins The sequence of amino acids in a protein dictates its three dimensional structure This structure gives proteins their functions. • Long chains of amino acids fold into threedimensional shapes in cells, which allows the protein to perform its specific functions. • When a protein is denatured, its shape has been disrupted and it may not be able to perform its function. Four Levels of Structure Proteins exhibit up to four levels of structure • Primary structure is the sequence of amino acids linked together in a protein • Secondary structures are helices and pleated sheets • Tertiary structure refers to complex foldings of the protein chain held together by disulfide bridges, hydrophobic/hydrophilic interactions, and other bonds • Quaternary structure is found where multiple protein chains are linked together Three Dimensional Structures The type, position, and number of amino acids determine the structure and function of a protein • Precise positioning of amino acid R groups leads to bonds that determine secondary and tertiary structure • Disruption of these bonds leads to denatured proteins and loss of function Nucleic Acids Nucleotides are the monomers of nucleic acid chains All nucleotides are made of three parts • Phosphate group • Five-carbon sugar • Nitrogen-containing base Molecules of Heredity Two types of polymers of nucleic acids • DNA (deoxyribonucleic acid) found in chromosomes • Carries genetic information needed for protein construction • RNA (ribonucleic acid) • Copies of DNA used directly in protein construction Molecules of Heredity Two types of nucleotides • Ribonucleotides (A, G, C, and U) found in RNA • Deoxyribonucleotides (A, G, C, and T) found in DNA Molecules of Heredity Each DNA molecule consists of two chains of nucleotides that form a double helix Other Nucleotides Nucleotides act as intracellular messengers Nucleotides act as energy carriers • Adenosine triphosphate (ATP) carries energy stored in bonds between phosphate groups Nucleotides as enzyme assistants 3.1 What Does The Plasma Membrane Do? The cell plasma membrane separates the cell contents from the external environment. The membrane acts as a gatekeeper, regulating the passage of molecules into and out of the cell. Plasma Membrane Functions of the plasma membrane • Isolates the cell’s contents from environment • Regulates exchange of essential substances • Communicates with other cells • Creates attachments within and between other cells • Regulates biochemical reactions Structure Of The Plasma Membrane Phospholipids are the basis of membrane structure • Polar, hydrophilic head • Two non-polar, hydrophobic tails CH3 CH2 CH2 CH2 CH2 CH2 CH2 – CH3 O + H3C N CH2 CH2 O P O CH2 O CH3 CH2 CH O HC O C CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH O H2C O C CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH3 tails (hydrophobic) head (hydrophilic) Fig. 3-2 The Phospholipid Bilayer Hydrophobic and hydrophilic interactions drive phospholipids into bilayers • • • • Double row of phospholipids Polar heads face outward and inward Non-polar tails mingle within the membrane Cholesterol in animal membranes keeps them flexible The Phospholipid Bilayer Individual phospholipid molecules are not bonded to one another Some of the phospholipids have unsaturated fatty acids, whose double bonds introduce “kinks” into their “tails” The above features make the membrane fluid Plasma Membrane as Gatekeeper The phospholipid bilayer blocks the passage of most molecules. The embedded proteins selectively transport, respond to, and recognize molecules. There are three types of membrane proteins— transport proteins, receptor proteins, and recognition proteins. Membrane Proteins Form a Mosaic Proteins are embedded in the phospholipid bilayer • • • Some proteins can float and drift Other proteins are anchored by protein filaments in the cytoplasm Many proteins have attached carbohydrates (glycoproteins) Movement of Molecules in Fluids Definitions relevant to substance movement • A fluid is a substance that can move or change shape in response to external forces • A solute is a substance that can be dissolved (dispersed as ions or molecules) in a solvent • A solvent is a fluid capable of dissolving a solute Movement of Molecules in Fluids Definitions relevant to substance movement (continued) • The concentration of molecules is the number of them in a given volume unit • A gradient is a physical difference in temperature, pressure, charge, or concentration in two adjacent regions Movement of Molecules in Fluids Why molecules move from one place to another • Substances move in response to a concentration gradient • Molecules move from high to low concentration (diffusion) until dynamic equilibrium is reached Movement of Molecules in Fluids The greater the concentration gradient, the faster the rate of diffusion Diffusion cannot move molecules rapidly over long distances 3.6 How Do Diffusion And Osmosis Affect Transport Across The Plasma Membrane? Concentration gradients of ions and molecules exist across the plasma membranes of all cells There are two types of movement across the plasma membrane • Passive transport • Energy-requiring transport Movement Across Membranes Passive transport • Substances move down their concentration gradients across a membrane • No energy is expended • Membrane proteins and phospholipids may limit which molecules can cross, but not the direction of movement Movement Across Membranes Energy-requiring transport • Substances are driven against their concentration gradients • Energy is expended Passive Transport Plasma membranes are selectively permeable • Different molecules move across at different locations and rates • A concentration gradient drives all three types of passive transport: simple diffusion, facilitated diffusion, and osmosis Passive Transport Simple diffusion • Lipid soluble molecules (e.g. vitamins A and E, gases) and very small molecules diffuse directly across the phospsholipid bilayer Passive Transport Facilitated diffusion • Water soluble molecules like ions, amino acids, and sugars diffuse with the aid of channel and carrier transport proteins Passive Transport Osmosis – the special case of water diffusion • Water diffuses from high concentration (high purity) to low concentration (low purity) across a membrane • Dissolved substances reduce the concentration of free water molecules (and hence the purity of water) in a solution • The flow of water across a membrane depends on the concentration of water in the internal or external solutions Passive Transport Comparison terms for solutions on either side of a membrane • Isotonic solutions have equal concentrations of water and equal concentrations of dissolved substances • No net water movement occurs across the membrane Passive Transport • A hypertonic solution is one with lower water concentration or higher dissolved particle concentration • Water moves across a membrane towards the hypertonic solution • A hypotonic solution is one with higher water concentration or lower dissolved particle concentration • Water moves across a membrane away from the hypotonic solution 3.7 How Do Molecules Move Against A Concentration Gradient? Energy-requiring transport processes • During active transport, the cell uses energy to move substances against a concentration gradient. • Membrane proteins regulate active transport. Active Transport Active-transport membrane proteins move molecules across using ATP • Proteins span the entire membrane • Often have a molecule binding site and an ATP binding site • Often referred to as pumps Endocytosis Cells import large particles or substances via endocytosis Plasma membrane pinches off to form a vesicle in endocytosis • Types of endocytosis • Pinocytosis • Receptor-mediated endocytosis • Phagocytosis Endocytosis Types of endocytosis • Pinocytosis (“cell drinking”) brings in droplet of extracellular fluid Endocytosis Types of endocytosis • Receptor-mediated endocytosis moves specific molecules into the cell Endocytosis Types of endocytosis • Phagocytosis (“cell eating”) moves large particles or whole organisms into the cell Exocytosis Exocytosis • Vesicles join the membrane, dumping out contents in exocytosis What Is the Cell Theory? Tenets of Modern Cell Theory • Every living organism is made of one or more cells • The smallest organisms are made of single cells while multicellular organisms are made of many cells • All cells arise from pre-existing cells 4.1 What Features Are Shared By All Cells? Cells are the smallest unit of life. Cells are enclosed by a plasma membrane. Cells use DNA as a hereditary blueprint. Cells contain cytoplasm, which is all the material inside the plasma membrane and outside the DNA-containing region. Cells obtain energy and nutrients from their environment. 4.1 What Features Are Shared By All Cells? Cell function limits cell size. • Most cells are small, ranging from 1 to 100 micrometers in diameter • Cells need to exchange nutrients and wastes with the environment • No part of the cell can be far away from the external environment Cell Function Limits Cell Size • Diffusion of molecules across cell membranes limits the diameter of cells. • As cells get bigger, their nutrient and waste elimination needs grow faster than the membrane area to accommodate them. tallest trees 10 m 1m 10 cm 1 cm adult human visible with unaided human eye Relative sizes Diameter 100 m chicken egg frog embryo 100 m 10 m 1 m Units of measurement: 1 meter (m) = 39.37 inches 1 centimeter (cm) = 1/100 m 1 millimeter (mm) = 1/1,000 m 1 micrometer (m) = 1/1,000,000 m 1 nanometer (nm) = 1/1,000,000,000 m 10 nm 1 nm 0.1 nm visible with special electron microscopes 100 nm most eukaryotic cells visible with conventional electron microscope visible with light microscope 1 mm mitochondrion most bacteria virus proteins diameter of DNA double helix atoms Fig. 4-1 All Cells Share Common Features A plasma membrane encloses all cells and regulates material flow Cytoplasm is the fluid interior where a cell’s metabolic reactions occur • Contains organelles • Fluid portion (cytosol) contains water, salts, and organic molecules All Cells Share Common Features All cells use DNA (deoxyribonucleic acid) as a hereditary blueprint All cells use RNA (ribonucleic acid) to copy DNA to make proteins All Cells Share Common Features All cells obtain energy and nutrients from the environment All cells use common building blocks to build the molecules of life Some Cell Types Have Cell Walls Stiff coatings on outer surfaces of bacteria, plants, fungi, and some protists are cell walls • • Cells walls support and protect fragile cells and are usually porous Cell walls are composed of polysaccharides like cellulose or chitin 4.2 How Do Prokaryotic And Eukaryotic Cells Differ? There are two kinds of cells. • Prokaryotic cells • Are found only in two groups of singlecelled organisms—the bacteria and archaea • Eukaryotic cells • Are structurally more complex cells • Possess a membrane-enclosed nucleus • Probably arose from prokaryotic cells Prokaryotic Cells No nuclear membrane or membranebound organelles present Some have internal membranes used to capture light Cytoplasm contains ribosomes used for protein synthesis Cytoplasm may contain food granules Prokaryotic Cells Much smaller than eukaryotic cells (< 5 µm long) Have a simple internal structure Surrounded by a stiff cell wall, which provides shape and protection Can take the shape of rods, spheres, or helices Prokaryotic Cells Some propelled by flagella Infectious bacteria may have polysaccharide adhesive capsules and slime layers on their surfaces Pili and fimbriae are protein projections in some bacteria that further enhance adhesion There Are Two Basic Cell Types Eukaryotic • True nucleus • Includes Protist, Fungi, Plant, and Animal cells 4.3 What Are The Main Features Of Eukaryotic Cells? Eukaryotic cells are > 10 µm long The cytoskeleton provides shape and organization A variety of membrane-enclosed organelles perform specific functions Major Features Nucleus: contains DNA Mitochondria: produce energy Endoplasmic reticulum: synthesizes membrane components and lipids Golgi apparatus: molecule sorting center Lysosomes: digest cellular membranes or defective organelles Microtubules: make up the cytoskeleton 4.4 What Role Does The Nucleus Play? The nucleus is the largest organelle in the cell. • It is bounded by a nuclear envelope. • It contains granular-looking chromatin. • It contains the nucleolus. The Nucleus The nuclear envelope separates chromosomes from cytoplasm • Envelope is a double membrane with nuclear pores for transport • Some smaller materials can move through the pores, while others, such as DNA, are excluded. • Outer membrane is studded with ribosomes The Nucleus The nucleus nuclear envelope nucleolus nuclear pores nucleus nuclear pores chromatin (a) Structure of the nucleus (b) Yeast cell Fig. 4-5 The Nucleus The nucleus contains DNA in various configurations • Compacted chromosomes (during cell division) • Diffuse chromatin (as DNA directs reactions through an RNA intermediate by coding for proteins) The Nucleus Darker area within the nucleus called the nucleolus • Functions as the site of ribosome synthesis • Ribosomes synthesize proteins • Ribosomes are composed of RNA and proteins 4.5 What Roles Do Membranes Play In Eukaryotic Cells? The plasma membrane isolates the cell, and alternately, helps it interact with its environment. • The phospholipid bilayer contains globular proteins that regulate the transport of molecules into and out of the cell. • Plant, fungi, and some protist cells also have a cell wall outside the plasma membrane, which acts as a protective coating. System of Membranes Vesicles are membranous sacs that transport substances among the separate regions of the membrane system System of Membranes The endoplasmic reticulum (ER) forms a series of enclosed, interconnected channels within cell • There are two forms of ER: • Rough endoplasmic reticulum: is studded with ribosomes • Smooth endoplasmic reticulum: has no ribosomes System of Membranes Smooth ER has no ribosomes • Contains enzymes that detoxify drugs (in liver cells) • Synthesizes phospholipids and cholesterol. • Together with rough ER are the sites of new membrane synthesis for the cell. System of Membranes Rough ER is studded with ribosomes on outside • Produces proteins and phospholipids destined for other membranes or for secretion (export) • Together with rough ER are the sites of new membrane synthesis for the cell. System of Membranes The Golgi Apparatus is a set of stacked flattened sacs • Receive proteins from ER (via transport vesicles) and sorts them by destination • Modify some molecules (e.g. proteins to glycoproteins) • Package material into vesicles for transport System of Membranes Three fates of substances made in the membrane system: 1. Secreted proteins made in RER, travel through Golgi, then are exported through plasma membrane 2. Digestive proteins made in RER, travel through Golgi, and are packaged as lysosomes for use in cell 3. Membrane proteins and lipids made in ER, travel through Golgi, and replenish or enlarge organelle and plasma membranes Vacuoles Fluid-filled sacs with a single membrane Functions of vacuoles • Contractile vacuoles in freshwater organisms used to collect and pump water out • Many plant cells have a large central vacuole. Mitochondria Extract Food Energy Function as the “powerhouses of the cell” • Mitochondria extract energy from food molecules • Extracted energy is stored in high-energy bonds of ATP • Energy extraction process involves anaerobic and aerobic reactions Major Features Animal and plant cells differ with regards to cell walls, chloroplasts, plastids, central vacuoles, and centrioles Chloroplasts Chloroplasts are specialized organelles to convert solar energy into sugars The thylakoid membranes in chloroplasts contain chlorophyll and other pigments that capture sunlight and make sugar, CO2, and water (photosynthesis) Plants Use Plastids for Storage Plastids found only in plants and photosynthetic protists Surrounded by a double membrane Functions • • Storage for photosynthetic products like starch Storage of pigment molecules giving color to ripe fruit Cytoskeleton The cytoskeleton provides shape, support, and movement. • All organelles in the cell do not float about the cytoplasm, but instead, are attached to a network of protein fibers called the cytoskeleton. Cilia and Flagella Functions • Cilia or flagella may be used to move cell about • Cilia may be used to create currents of moving fluid in their environment