* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 06_Metabolism of lipid
Peptide synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Microbial metabolism wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Proteolysis wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Human digestive system wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Butyric acid wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Citric acid cycle wikipedia , lookup
Biosynthesis wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
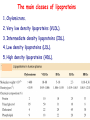
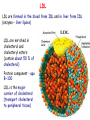
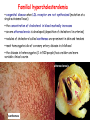
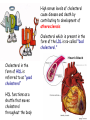
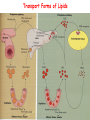
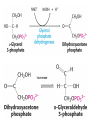
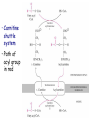
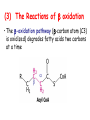
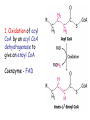
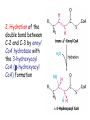
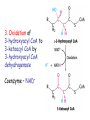
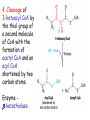
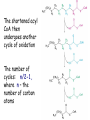
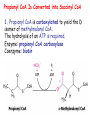
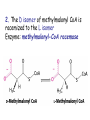
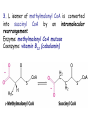
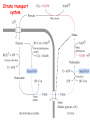
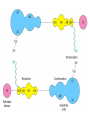
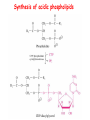
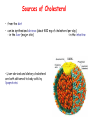
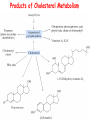
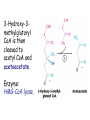
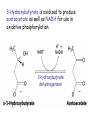
METABOLISM OF LIPIDS: PHYSIOLOGICAL ROLE OF LIPIDS Energetic role (fuel molecules) Components of membranes (structural role) Precursors for many hormones (steroids) Signal molecules (prostaglandins) Protective role (lipids surround important organs) Enzyme cofactors (vitamin K) Electron carriers (ubiquinone) Insulation against temperature extremes DIGESTION OF DIETARY LIPIDS Lipids in diet: triacylglycerols phospholipids cholesterol Digestion – in small intestine. Enzyme – pancreatic lipase. Lipase catalyzes hydrolysis at the C1 and C3 positions of TGs producing free fatty acids and 2-monoacylglycerol. Colipase – protein which is present in the intestine and helps bind the water-soluble lipase to the lipid substrates. Colipase also activates lipase. Bile salts (salts of bile acids) are required for lipids digestion. Bile salts are synthesized in the liver from cholesterol. Taurocholate and glycocholate - the most abundant bile salts. Amphipathic: hydrophilic (blue) and hydrophobic (black) TGs are water insoluble and lipase is water soluble. Digestion of TGs takes place at lipid-water interfaces. Rate of digestion depends on the surface area of the interface. Bile salts are amphipathic, they act as detergent emulsifying the lipid drops and increasing the surface area of the interface. Bile salts also activates the lipase. Inadequate production of bile salts results in steatorrhea. ABSORPTION OF DIETARY LIPIDS Lipid absorption – passive diffusion process. 2-monoacylglycerols, fatty acids, lysophosphoglycerides, free cholesterol form micelles with bile salts. TRANSPORT FORMS OF LIPIDS • TGs, cholesterol and cholesterol esters are insoluble in water and cannot be transported in blood or lymph as free molecules • These lipids assemble with phospholipids and apoproteins (apolipoproteins) to form spherical particles called lipoprotein Structure: Hydrophobic core: -TGs, -cholesteryl esters Hydrophilic surfaces: -cholesterol, -phospholipids, -apolipoproteins The main classes of lipoproteins 1.Chylomicrons. 2.Very low density lipoproteins (VLDL). 3.Intermediate density lipoproteins (IDL). 4.Low density lipoproteins (LDL). 5.High density lipoproteins (HDL). Chylomicrons • are the largest lipoproteins (180 to 500 nm in diameter) • are synthesized in the ER of intestinal cells • contain 85 % of TGs (it is the main transport form of dietary TGs). • apoprotein B-48 (apo B-48) is the main protein component • deliver TGs from the intestine (via lymph and blood) to tissues (muscle for energy, adipose for storage). • bind to membrane-bound lipoprotein lipase (at adipose tissue and muscle), where the triacylglycerols are again degraded into free fatty acids and monoacylglycerol for transport into the tissue • are present in blood only after feeding exocytosis Lymphatic vessel • are formed in the liver VLDL • contain 50 % of TGs and 22 % of cholesterol • two lipoproteins — apo B-100 and apo E • the main transport form of TGs synthesized in the organism (liver) • deliver the TGs from liver to peripheral tissue (muscle for energy, adipose for storage) • bind to membrane-bound lipoprotein lipases (triacylglycerols are again degraded into free fatty acids and monoacylglycerol) triacylglycerol cholesteryl esters Apo B Apo E cholesterol phospholipids Lipoproteinlipase – enzyme which is located within capillaries of muscles and adipose tissue Function: hydrolyses of TGs of chylomicrons and VLDL. Formed free fatty acids and glycerol pass into the cells Chylomicrons and VLDL which gave up TGs are called remnants of chylomicrons and remnants of VLDL Remnants are rich in cholesterol esters Remnants of chylomicrons are captured by liver Remnants of VLDL are also called intermediate density lipoproteins (IDL) Fate of the IDL: - some are taken by the liver - others are degraded to the low density lipoproteins (LDL) (by the removal of more triacylglycerol) LDL LDL are formed in the blood from IDL and in liver from IDL (enzyme – liver lipase) LDL are enriched in cholesterol and cholesteryl esters (contain about 50 % of cholesterol) Protein component - apo B-100 LDL is the major carrier of cholesterol (transport cholesterol to peripheral tissue) Familial hypercholesterolemia congenital disease when LDL receptor are not synthesized (mutation at a single autosomal locus) the concentration of cholesterol in blood markedly increases severe atherosclerosis is developed (deposition of cholesterol in arteries) nodules of cholesterol called xanthomas are prominent in skin and tendons most homozygotes die of coronary artery disease in childhood the disease in heterozygotes (1 in 500 people) has a milder and more variable clinical course atherosclerosis xanthomas HDL are formed in the liver and partially in small intestine contain the great amount of proteins (about 40 %) pick up the cholesterol from peripheral tissue, chylomicrons and VLDL enzyme acyltransferase in HDL esterifies cholesterols, convert it to cholesterol esters and transport to the liver High serum levels of cholesterol cause disease and death by contributing to development of atherosclerosis Cholesterol which is present in the form of the LDL is so-called "bad cholesterol." Cholesterol in the form of HDL is referred to as "good cholesterol” HDL functions as a shuttle that moves cholesterol throughout the body Transport Forms of Lipids Storage and Mobilization of Fatty Acids (FA) • TGs are delivered to adipose tissue in the form of chylomicrones and VLDL, hydrolyzed by lipoprotein lipase into fatty acids and glycerol, which are taken up by adipocytes. • Then fatty acids are reesterified to TGs. • TGs are stored in adipocytes. • To supply energy demands fatty acids and glycerol are released – mobilisation of TGs. adipocyte •Lipolysis - hydrolysis of triacylglycerols by lipases. •A hormone-sensitive lipase converts TGs to free fatty acids and monoacylglycerol •Monoacylglycerol is hydrolyzed to fatty acid and glycerol or by a hormone-sensitive lipase or by more specific and more active monoacylglycerol lipase Transport of Fatty Acids and Glycerol • Fatty acids and glycerol diffuse through the adipocyte membrane and enter bloodstream. • Glycerol is transported via the blood in free state and oxidized or converted to glucose in liver. • Fatty acids are traveled bound to albumin. • In heart, skeletal muscles and liver they are oxidized with energy release. Oxidation of Glycerol Glycerol is absorbed by the liver. Steps: phosphorylation, oxidation and isomerisation. Glyceraldehyde 3-phosphate is an intermediate in: glycolytic pathway gluconeogenic pathways Isomerase Stages of fatty acid oxidation (1) Activation of fatty acids takes place on the outer mitochondrial membrane (2) Transport into the mitochondria (3) Degradation to two-carbon fragments (as acetyl CoA) in the mitochondrial matrix (b-oxidation pathway) (1) Activation of Fatty Acids • Fatty acids are converted to CoA thioesters by acyl-CoA synthetase (ATP dependent) • The PPi released is hydrolyzed by a pyrophosphatase to 2 Pi • Two phosphoanhydride bonds (two ATP equivalents) are consumed to activate one fatty acid to a thioester (2) Transport of Fatty Acyl CoA into Mitochondria • The carnitine shuttle system. • Fatty acyl CoA is first converted to acylcarnitine (enzyme carnitine acyltransferase I (bound to the outer mitochondrial membrane). • Acylcarnitine enters the mitochondria by a translocase. • The acyl group is transferred back to CoA (enzyme carnitine acyltransferase II). • Carnitine shuttle system • Path of acyl group in red (3) The Reactions of b oxidation • The b-oxidation pathway (b-carbon atom (C3) is oxidized) degrades fatty acids two carbons at a time b 1. Oxidation of acyl CoA by an acyl CoA dehydrogenase to give an enoyl CoA Coenzyme - FAD 2. Hydration of the double bond between C-2 and C-3 by enoyl CoA hydratase with the 3-hydroxyacyl CoA (b-hydroxyacyl CoA) formation 3. Oxidation of 3-hydroxyacyl CoA to 3-ketoacyl CoA by 3-hydroxyacyl CoA dehydrogenase Coenzyme – NAD+ 4. Cleavage of 3-ketoacyl CoA by the thiol group of a second molecule of CoA with the formation of acetyl CoA and an acyl CoA shortened by two carbon atoms. Enzyme b-ketothiolase. The shortened acyl CoA then undergoes another cycle of oxidation The number of cycles: n/2-1, where n – the number of carbon atoms b-Oxidation of Fatty acyl CoA saturated fatty acids • One round of b oxidation: 4 enzyme steps produce acetyl CoA from fatty acyl CoA • Each round generates one molecule each of: FADH2 NADH Acetyl CoA Fatty acyl CoA (2 carbons shorter each round) Fates of the products of b-oxidation: - NADH and FADH2 - are used in ETC - acetyl CoA - enters the citric acid cycle - acyl CoA – undergoes the next cycle of oxidation ATP Generation from Fatty Acid Oxidation Net yield of ATP per one oxidized palmitate Palmitate (C15H31COOH) - 7 cycles – n/2-1 • The balanced equation for oxidizing one palmitoyl CoA by seven cycles of b oxidation Palmitoyl CoA + 7 HS-CoA + 7 FAD+ + 7 NAD+ + 7 H2O 8 Acetyl CoA + 7FADH2 + 7 NADH + 7 H+ ATP generated 8 acetyl CoA 7 FADH2 7 NADH 10x8=80 7x1.5=10.5 7x2.5=17.5 108 ATP ATP expended to activate palmitate Net yield: -2 106 ATP Propionyl CoA Is Converted into Succinyl CoA 1. Propionyl CoA is carboxylated to yield the D isomer of methylmalonyl CoA. The hydrolysis of an ATP is required. Enzyme: propionyl CoA carboxylase Coenzyme: biotin 2. The D isomer of methylmalonyl CoA is racemized to the L isomer Enzyme: methylmalonyl-CoA racemase 3. L isomer of methylmalonyl CoA is converted into succinyl CoA by an intramolecular rearrangement Enzyme: methylmalonyl CoA mutase Coenzyme: vitamin B12 (cobalamin) Fatty Acid Synthesis • Occurs mainly in liver and adipocytes, in mammary glands during lactation • Occurs in cytoplasm • FA synthesis and degradation occur by two completely separate pathways • When glucose is plentiful, large amounts of acetyl CoA are produced by glycolysis and can be used for fatty acid synthesis A. Transport of Acetyl CoA to the Cytosol • Acetyl CoA from catabolism of carbohydrates and amino acids is exported from mitochondria via the citrate transport system • Cytosolic NADH also converted to NADPH • Two molecules of ATP are expended for each round of this cyclic pathway Citrate transport system B. Carboxylation of Acetyl CoA Enzyme: acetyl CoA carboxylase Prosthetic group - biotin A carboxybiotin intermediate is formed. ATP is hydrolyzed. The CO2 group in carboxybiotin is transferred to acetyl CoA to form malonyl CoA. Acetyl CoA carboxylase is the regulatory enzyme. C. The Reactions of Fatty Acid Synthesis • Five separate stages: (1) Loading of precursors via thioester derivatives (2) Condensation of the precursors (3) Reduction (4) Dehydration (5) Reduction Final reaction of FA synthesis • Rounds of synthesis continue until a C16 palmitoyl group is formed • Palmitoyl-ACP is hydrolyzed by a thioesterase Overall reaction of palmitate synthesis from acetyl CoA and malonyl CoA Acetyl CoA + 7 Malonyl CoA + 14 NADPH + 14 H+ Palmitate + 7 CO2 + 14 NADP+ + 8 HS-CoA + 6 H2O THE CONTROL OF FATTY ACID METABOLISM Acetyl CoA carboxylase plays an essential role in regulating fatty acid synthesis and degradation. The carboxylase is controlled by hormones: glucagon, epinephrine, and insulin. Another regulatory factors: citrate, palmitoyl CoA, and AMP Global Regulation is carried out by means of reversible phosphorylation Acetyl CoA carboxylase is switched off by phosphorylation and activated by dephosphorylation Insulin stimulates fatty acid synthesis causing dephosphorylation of carboxylase. Glucagon and epinephrine have the reverse effect (keep the carboxylase in the inactive phosphorylated state). Protein kinase is activated by AMP and inhibited by ATP. Carboxylase is inactivated when the energy charge is low. Local Regulation Acetyl CoA carboxylase is allosterically stimulated by citrate. The level of citrate is high when both acetyl CoA and ATP are abundant (isocitrate dehydrogenase is inhibited by ATP). Palmitoyl CoA inhibits carboxylase. Synthesis of Triacylglycerols (TGs) and Glycerophospholipids (GPLs) Glycerol 3-phosphate can be obtained either by the reduction of dihydroxyecetone phosphate (primarily) or by the phosphorylation of glycerol (to a lesser extent). Synthesis of acidic phospholipids Functions of Cholesterol • a precursor of steroid hormones (progesterone, testosterone, estradiol, cortisol, etc.) • a precursor of bile acids • a precursor of vitamin D • important component of many mammalian membranes (modulates the fluidity) Sources of Cholesterol • from the diet • can be synthesized de novo (about 800 mg of cholesterol per day) - in the liver (major site) - in the intestine • Liver-derived and dietary cholesterol are both delivered to body cells by lipoproteins Synthesis of Cholesterol Three stages of cholesterol biosynthesis 1. Synthesis of isopentenyl pyrophosphate, that is the key building block of cholesterol, from acetyl CoA 2. Condensation of six molecules of isopentenyl pyrophosphate to form squalene 3. Squalene cyclizes and the tetracyclic product is converted into cholesterol Acetyl CoA (C2) Isopentenyl pyrophosphate (C5) Squalene (C30) Cholesterol (C27) THE REGULATION OF CHOLESTEROL BIOSYNTHESIS Regulatory enzyme - 3-hydroxy-3-methylglutaryl CoA reductase. Tetrameric enzyme. NADPH coenzyme HMG CoA reductase is controlled in multiple ways: 1. The rate of synthesis of reductase mRNA is controlled by the sterol regulatory element binding protein (SREBP). When cholesterol levels fall this protein migrates to the nucleus and enhance transcription. 2. The rate of translation of reductase mRNA is inhibited by cholesterol 3. The degradation of the reductase is controlled. The increase of cholesterol concentration makes the enzyme more susceptible to proteolysis. 4. Phosphorylation decreases the activity of the reductase. Enzyme is switched off by an AMP-activated protein kinase. Thus, cholesterol synthesis ceases when the ATP level is low. Products of Cholesterol Metabolism ATHEROSCLEROSIS The desirable level of cholesterol in blood plasma: < 200 mg/dl (< 5 mmol/l) For a healthy person, the LDL/HDL ratio is 3.5 KETONE BODIES The entry of acetyl CoA into the citric acid cycle depends on the availability of oxaloacetate. The concentration of oxaloacetate is lowered if carbohydrate is unavailable (starvation) or improperly utilized (diabetes). Oxaloacetate is normally formed from pyruvate by pyruvate carboxylase (anaplerotic reaction). Fats burn in the flame of carbohydrates. In fasting or diabetes the gluconeogenesis is activated and oxaloacetate is consumed in this pathway. Fatty acids are oxidized producing excess of acetyl CoA which is converted to ketone bodies: b-Hydroxybutyrate Acetoacetate Acetone Ketone bodies are synthesized in liver mitochondria and exported to different organs. Ketone bodies are fuel molecules (can fuel brain and other cells during starvation) A. Synthesis of ketone bodies Two molecules of acetyl CoA condense to form acetoacetyl CoA. Enzyme – thiolase. Acetoacetyl CoA reacts with acetyl CoA and water to give 3hydroxy-3methylglutaryl CoA (HMGCoA) and CoA. Enzyme: HMG-CoA synthase 3-Hydroxy-3methylglutaryl CoA is then cleaved to acetyl CoA and acetoacetate. Enzyme: HMG-CoA lyase. 3-Hydroxybutyrate is formed by the reduction of acetoacetate by 3-hydroxybutyrate dehydrogenase. Acetoacetate also undergoes a slow, spontaneous decarboxylation to acetone. The odor of acetone may be detected in the breath of a person who has a high level of acetoacetate in the blood. B. Ketone bodies are a major fuel in some tissues Ketone bodies diffuse from the liver mitochondria into the blood and are transported to peripheral tissues. Ketone bodies are important molecules in energy metabolism. Heart muscle and the renal cortex use acetoacetate in preference to glucose in physiological conditions. The brain adapts to the utilization of acetoacetate during starvation and diabetes. 3-Hydroxybutyrate is oxidized to produce acetoacetate as well as NADH for use in oxidative phosphorylation. 3-hydroxybutyrate dehydrogenase Acetoacetate is activated by the transfer of CoA from succinyl CoA in a reaction catalyzed by a specific CoA transferase. Acetoacetyl CoA is cleaved by thiolase to yield two molecules of acetyl CoA (enter the citric acid cycle). CoA transferase is present in all tissues except liver. Ketone bodies are a watersoluble, transportable form of acetyl units KETOSIS The absence of insulin in diabetes mellitus liver cannot absorb glucose inhibition of glycolysis activation of gluconeogenesis deficit of oxaloacetate activation of fatty acid mobilization by adipose tissue large amounts of acetyl CoA which can not be utilized in Krebs cycle large amounts of ketone bodies (moderately strong acids) severe acidosis (ketosis) Impairment of the tissue function, most importantly in the central nervous system