* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download NMDA and stroke
Programmed cell death wikipedia , lookup
Purinergic signalling wikipedia , lookup
Biochemical cascade wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Leukotriene B4 receptor 2 wikipedia , lookup
Glutamate receptor wikipedia , lookup
Paracrine signalling wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Signal transduction wikipedia , lookup
VLDL receptor wikipedia , lookup
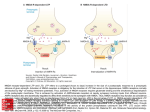
PHM142 Fall 2016 Coordinator: Dr. Jeffrey Henderson Instructor: Dr. David Hampson STROKE THERAPIES & THE NMDA RECEPTOR Michael Haichin Matin Shariatian Roman Pelyavskyy Niraj Maulkhan CEREBROVASCULAR ACCIDENTS Strokes Hemorrhagic Ischemic • Bleeding within the brain • Interrupt blood supply to a • Resulting from blood vessel region of the brain rupture • Caused by either thrombus • Malformed or high blood or embolus pressure • 87% of strokes are of the ischemic type THE NMDA RECEPTOR (NMDAR) • N-methyl-D-aspartate receptor • Ion channel • Found in Nerve cells • Allows positively charged ions through the cell membrane • Has important roles in synaptic cell death following a stroke MECHANISM OF ACTION • Has binding site for glutamate and glycine • Is voltage dependent • Mg2+ ions block the channel pore • Sufficient depolarization will dislodge the magnesium ion and allow for Ca2+ and Na+ permeability • Therefore both the presence of glutamate (and glycine) and sufficient depolarization are required • Has allosteric sites which allow for potentiation NMDAR AND CELL DEATH FOLLOWING STROKE Stroke Interruption of blood supply to the brain Oxygen and Glucose depletion Na+/K+ transporters stop functioning Neurons become depolarized Glutamate is released NMDAR is activated and Ca2+ and Na+ enter the cell NMDAR AND CELL DEATH • Overload of Sodium and calcium in the cell causes water absorption • This attracts microglial cells and makes them phagocytic • Additionally overload of calcium can trigger several downstream lethal pathways • Nitrosative stress • Oxidative stress • Mitochondrial dysfunction (produces free radicals and induces apoptosis) • NMDAR is therefore an important drug target FAILED NMDAR STROKE THERAPIES PROPOSED TREATMENT • It was theorized that high concentrations of Glutamate served only to destroy neurons • Competitive and Non-competitive Antagonists for Glycine and Glutamate • THEORY: Blocking these NMDA Receptors entirely should just fix the problem of neuronal cell death, right? WHERE IT WENT WRONG • Competitive Antagonists would block healthy brain regions first • Inhibition of normal NMDAR functioning leads to unwanted side effects • Drowsiness • Hallucinations • Coma • NMDAR antagonism hinders endogenous, beneficial mechanisms • Plasticity • Long-term neuronal survival • Neurodevelopment (through Brain Derived Neurotrophic Factor) • Toxicity in humans • Unable to replicate drug levels in animal models that lead to neuroprotection DEVELOPMENT OF NEW THERAPIES BUT WAIT THERE’S HOPE! • Target downstream effects of the NMDAR rather than blocking all of the NMDAR activity • In vivo and In vitro studies have had success with this approach • Ex. Inactivation of PSD-95, a scaffolding protein that binds NMDARs to neuronal nitric oxide synthase (nNOS) THE ROLE OF PSD-95 • PSD-95 is a scaffolding protein that binds NMDARs to neuronal nitric oxide synthase (nNOS) • The calcium influx from the over activated NMDA receptor causes the activation of nNOS • Active nNOS results in the production of nitric oxide (NO), a signaling molecule that mediates NMDAR-dependent excitotoxicity INACTIVATION ON PSD-95 • PSD-95 deletion/inhibition prevents NMDAR activity from producing NO and thus suppresses excitotoxicity • NMDAR activity is unaffected by mutating PSD-95 in vivo (no unwanted side effects of blocking NMDA activity) • Drugs are now being produced to inhibit the PSD95 signal pathway, thus preventing brain damage resulting from stroke mediated by NO SUMMARY • Two major types of strokes: • Hemorrhagic strokes are caused by bleeding within the brain, resulting from a ruptured blood vessel. • Ischemic strokes interrupt the blood supply to a region of the brain. • NMDAR is an ion channel activate by glutamate and glycine binding • Simultaneous depolarization is required to remove Mg+ ion from blocking its pore • It is involved in cell death following stroke by its over activation due to excessive glutamate released by depolarized cells. • This causes Na+ and Ca2+ overload in the cells leading to swelling which attracts microglial cells • Ca2+ overload also induces downstream lethal pathways that lead to free radical production and apoptosis SUMMARY • Complete inhibition of NMDAR leads to: • Damage to healthy brain regions • Unwanted side effects including drowsiness and hallucinations • Inhibition of endogenous, beneficial mechanisms • Early NMDAR antagonists produce low-dose toxicity in humans • PSD-95 is a scaffolding protein that binds NMDARs to neuronal nitric oxide synthase (nNOS), • nNOS is stimulated by the calcium influx from the over activated NMDA receptor resulting in the production of NO, which can cause neuronal cell death • PSD-95 deletion/inhibition prevents NMDAR activity from producing NO and thus suppresses excitotoxicity without adverse side effects REFERENCES 1. Aarts M, Liu Y, Liu L, et al: Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science 298. (2002): 846850 2. Carlson, Neil R. Physiology of Behaviour. Harlow: Pearson/Education, 2012. 3. Dawson,V. et al. "Resistance to Neurotoxicity in Cortical Cultures from Neuronal Nitric Oxide Synthase-Deficient Mice." The Journal of Neuroscience 16.8 (1996): 2479-487 4. Hoyte, L., P. Barber, A. Buchan, and M. Hill. "The Rise and Fall of NMDA Antagonists for Ischemic Stroke." CMM Current Molecular Medicine 4.2 (2004): 131-36.Web. 5. Ikonomidou, Chrysanthy, and Lechoslaw Turski. "Why Did NMDA Receptor Antagonists Fail Clinical Trials for Stroke and Traumatic Brain Injury?" The Lancet Neurology 1.6 (2002): 383-86. Web. 6. Lipton, Stuart A. “Failures and Successes of NMDA Receptor Antagonists: Molecular Basis for the Use of Open-Channel Blockers like Memantine in the Treatment of Acute and Chronic Neurologic Insults.” NeuroRx 1.1 (2004): 101–110. Print. 7. Martin, H and Yu,T. "Blocking the Deadly Effects of the NMDA Receptor in Stroke."Cell 140.2 (2010): 174-76 8. Moskowitz, Michael A., Eng H. Lo, and Constantino Iadecola. “The Science of Stroke: Mechanisms in Search of Treatments.” Neuron 67.2 (2010): 181-98. 9. Ogden, Kevin K., and Stephen F. Traynelis. "New Advances in NMDA Receptor Pharmacology." Trends in Pharmacological Sciences 32.12 (2011): 726-33. Web. 10. Shohami, Esther, and Anat Biegon. "Novel Approach to the Role of NMDA Receptors in Traumatic Brain Injury." CNS & Neurological Disorders - Drug Targets 13.4 (2014): 567-73. PubMed. Web. 11. Tu W., et al. "DAPK1 Interaction with NMDA Receptor NR2B Subunits Mediates Brain Damage in Stroke." Cell 140.2 (2010): 222-34.Web. 12. Vandongen, Antonius, and Marie Blanke. "Activation Mechanisms of the NMDA Receptor." Biology of the NMDA Receptor Frontiers in Neuroscience (2008): 283-312.Web. 13. Zhou, Li et al. "Treatment of Cerebral Ischemia by Disrupting Ischemia-induced Interaction of NNOS with PSD-95." Nature Medicine 16.12 (2010): 1439-443