* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download energy - Old Saybrook Public Schools
Survey
Document related concepts
Fatty acid metabolism wikipedia , lookup
Metalloprotein wikipedia , lookup
Metabolic network modelling wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Photosynthesis wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Electron transport chain wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Microbial metabolism wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Biochemistry wikipedia , lookup
Citric acid cycle wikipedia , lookup
Transcript
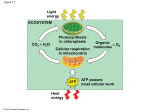
6 Pathways that Harvest and Store Chemical Energy Introduction to Metabolism: Energetics • Energy is stored in chemical bonds and can be released and transformed by metabolic pathways. • Chemical energy available to do work is termed free energy (G). • In cells, energy-transforming reactions are often coupled: • An energy-releasing (exergonic) reaction is coupled to an energy-requiring (endergonic) reaction. Concept 6.1 ATP, Reduced Coenzymes, and Chemiosmosis Play Important Roles in Biological Energy Metabolism Five principles governing metabolic pathways: 1. Chemical transformations occur in a series of intermediate reactions that form a metabolic pathway. 2. Each reaction is catalyzed by a specific enzyme. 3. Most metabolic pathways are similar in all organisms. 4. In eukaryotes, many metabolic pathways occur inside specific organelles. 5. Each metabolic pathway is controlled by enzymes that can be inhibited or activated Figure 6.1 The Concept of Coupling Reactions ATP – The “Power Molecule” Adenosine triphosphate (ATP) is a kind of “energy currency” in cells. Energy released by exergonic reactions is stored in the bonds of ATP. When ATP is hydrolyzed, free energy is released to drive endergonic reactions. ATP H 2O ADP Pi freeenergy Hydrolysis of ATP is exergonic Hydrolysis of ATP is exergonic ΔG is about –7.3 kcal • Free energy of the bond between phosphate groups is much higher than the energy of the O—H bond that forms after hydrolysis. • Phosphate groups are negatively charged, so energy is required to get them near enough to each other to make the covalent bonds in the ATP molecule. • ATP can be formed by substrate-level phosphorylation or oxidative phosphorylation Redox Reactions Energy can also be transferred by the transfer of electrons in oxidation–reduction, or redox reactions. • Reduction is the gain of one or more electrons. • Oxidation is the loss of one or more electrons. Redox Reactions Oxidation and reduction always occur together. Redox Reactions • Transfers of hydrogen atoms involve transfers of electrons (H = H+ + e–). • When a molecule loses a hydrogen atom, it becomes oxidized. • The more reduced a molecule is, the more energy is stored in its bonds. • Energy in the reducing agent is transferred to the reduced product. Redox Reactions Coenzymes – electron carriers Coenzyme NAD+ is a key electron carrier in redox reactions. NAD+ (oxidized form- lost electrons) NADH (reduced form – gained electrons) Figure 6.4 A NAD+/NADH Is an Electron Carrier in Redox Reactions Coenzymes Reduction of NAD+ is highly endergonic: NAD H 2e NADH Oxidation of NADH is highly exergonic: NADH H 1 2 O2 NAD H 2O Figure 6.4 B NAD+/NADH Is an Electron Carrier in Redox Reactions Oxidative Phosphorylation • In cells, energy is released in catabolism by oxidation and trapped by reduction of coenzymes such as NADH. • Energy for anabolic processes is supplied by ATP. Oxidative phosphorylation transfers energy from NADH to ATP. REMEMBER: Catabolism – breaking down large to small Anabolism – making small to large Oxidative phosphorylation Oxidative phosphorylation couples oxidation of NADH: NADH NAD H 2e energy with production of ATP: energy ADP Pi ATP Chemiosmosis The coupling is chemiosmosis—diffusion of protons across a membrane, which drives the synthesis of ATP. Chemiosmosis converts potential energy of a proton gradient across a membrane into the chemical energy in ATP. Figure 6.5 A Chemiosmosis Concept 6.1 ATP, Reduced Coenzymes, and Chemiosmosis Play Important Roles in Biological Energy Metabolism ATP synthase—membrane protein with two subunits: F0 is the H+ channel; potential energy of the proton gradient drives the H+ through. F1 has active sites for ATP synthesis. Figure 6.5 B Chemiosmosis Concept 6.1 ATP, Reduced Coenzymes, and Chemiosmosis Play Important Roles in Biological Energy Metabolism Chemiosmosis can be demonstrated experimentally. A proton gradient can be introduced artificially in chloroplasts or mitochondria in a test tube. ATP is synthesized if ATP synthase, ADP, and inorganic phosphate are present. *** Animated Tutorial 6.1 – BIOPORTAL*** Cellular Respiration Quick Overview – Cellular Respiration and Photosynthesis Cellular respiration is a major catabolic pathway. Glucose is oxidized: carbohydra te 6O2 6CO2 6H 2O chemical energy Photosynthesis is a major anabolic pathway. Light energy is converted to chemical energy: 6CO2 6H 2O light energy 6O2 carbohydra te Figure 6.7 ATP, Reduced Coenzymes, and Metabolism Concept 6.2 Carbohydrate Catabolism in the Presence of Oxygen Releases a Large Amount of Energy Cellular Respiration A lot of energy is released when reduced molecules with many C—C and C—H bonds are fully oxidized to CO2. Oxidation occurs in a series of small steps in three pathways: 1. glycolysis 2. pyruvate oxidation 3. citric acid cycle Figure 6.8 Energy Metabolism Occurs in Small Steps Figure 6.9 Energy-Releasing Metabolic Pathways Concept 6.2 Carbohydrate Catabolism in the Presence of Oxygen Releases a Large Amount of Energy Glycolysis: ten reactions. • Takes place in the cytosol. • Six-carbon glucose is converted to two threecarbon pyruvate • Final products (NET GAIN): 2 molecules of pyruvate (pyruvic acid) 2 molecules of ATP (“use 2 to get 2”) 2 molecules of NADH Figure 6.10 Glycolysis Converts Glucose into Pyruvate (Part 1) Endergonic reactions, requiring energy from ATP Figure 6.10 Glycolysis Converts Glucose into Pyruvate (Part 2) 6-carbon molecule is cleaved into two 3-carbon molecules Figure 6.10 Glycolysis Converts Glucose into Pyruvate (Part 3) Exergonic reactions, resulting in ATP and NADH being produced Concept 6.2 Carbohydrate Catabolism in the Presence of Oxygen Releases a Large Amount of Energy Examples of reaction types common in metabolic pathways: Step 6: Oxidation–reduction Step 7: Substrate-level phosphorylation Step 6 text art pg 107 here Step 7 Pyruvate Oxidation – aka Pyruvate Fixation Pyruvate Oxidation: Allows for the Citric Acid Cycle to occur in the mitochondrion Products: CO2 and acetate; acetate is then bound to coenzyme A (CoA) Concept 6.2 Carbohydrate Catabolism in the Presence of Oxygen Releases a Large Amount of Energy Citric Acid Cycle: 8 reactions, operates twice for every glucose molecule that enters glycolysis. Starts with Acetyl CoA; acetyl group is oxidized to two CO2. Oxaloacetate is regenerated in the last step. Figure 6.11 The Citric Acid Cycle Concept 6.2 Carbohydrate Catabolism in the Presence of Oxygen Releases a Large Amount of Energy Final reaction of citric acid cycle: Concept 6.2 Carbohydrate Catabolism in the Presence of Oxygen Releases a Large Amount of Energy Electron transport/ATP Synthesis: NADH is reoxidized to NAD+ and O2 is reduced to H2O in a series of steps. Respiratory chain—series of redox carrier proteins embedded in the inner mitochondrial membrane. Electron transport—electrons from the oxidation of NADH and FADH2 pass from one carrier to the next in the chain. Figure 6.12 Electron Transport and ATP Synthesis in Mitochondria Concept 6.2 Carbohydrate Catabolism in the Presence of Oxygen Releases a Large Amount of Energy The oxidation reactions are exergonic; the energy is used to actively transport H+ ions out of the mitochondrial matrix, setting up a proton gradient. ATP synthase in the membrane uses the H+ gradient to synthesize ATP by chemiosmosis. Concept 6.2 Carbohydrate Catabolism in the Presence of Oxygen Releases a Large Amount of Energy About 32 molecules of ATP are produced for each fully oxidized glucose. The role of O2: most of the ATP produced is formed by oxidative phosphorylation, which is due to the reoxidation of NADH. Concept 6.3 Carbohydrate Catabolism in the Absence of Oxygen Releases a Small Amount of Energy Under anaerobic conditions, NADH is reoxidized by fermentation. There are many different types of fermentation, but all operate to regenerate NAD+. The overall yield of ATP is only two—the ATP made in glycolysis. Concept 6.3 Carbohydrate Catabolism in the Absence of Oxygen Releases a Small Amount of Energy Lactic acid fermentation: End product is lactic acid (lactate). NADH is used to reduce pyruvate to lactic acid, thus regenerating NAD+. Figure 6.13 A Fermentation Concept 6.3 Carbohydrate Catabolism in the Absence of Oxygen Releases a Small Amount of Energy Alcoholic fermentation: End product is ethyl alcohol (ethanol). Pyruvate is converted to acetaldehyde, and CO2 is released. NADH is used to reduce acetaldehyde to ethanol, regenerating NAD+ for glycolysis. Figure 6.13 B Fermentation Concept 6.4 Catabolic and Anabolic Pathways Are Integrated Metabolic pathways are linked. Carbon skeletons (molecules with covalently linked carbon atoms) can enter catabolic or anabolic pathways. Figure 6.14 Relationships among the Major Metabolic Pathways of the Cell Concept 6.4 Catabolic and Anabolic Pathways Are Integrated Catabolism: Polysaccharides are hydrolyzed to glucose, which enter glycolysis. Lipids break down to fatty acids and glycerol. Fatty acids can be converted to acetyl CoA. Proteins are hydrolyzed to amino acids that can feed into glycolysis or the citric acid cycle. Concept 6.4 Catabolic and Anabolic Pathways Are Integrated Anabolism: Many catabolic pathways can operate in reverse. Gluconeogenesis—citric acid cycle and glycolysis intermediates can be reduced to form glucose. Acetyl CoA can be used to form fatty acids. Some citric acid intermediates can form nucleic acids. Concept 6.4 Catabolic and Anabolic Pathways Are Integrated Amounts of different molecules are maintained at fairly constant levels—the metabolic pools. This is accomplished by regulation of enzymes— allosteric regulation, feedback inhibition. Enzymes can also be regulated by altering the transcription of genes that encode the enzymes.