* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Introduction - Cedar Crest College
Cellular differentiation wikipedia , lookup
NMDA receptor wikipedia , lookup
Cell membrane wikipedia , lookup
Purinergic signalling wikipedia , lookup
Protein moonlighting wikipedia , lookup
Hedgehog signaling pathway wikipedia , lookup
Cytokinesis wikipedia , lookup
Endomembrane system wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Biochemical cascade wikipedia , lookup
List of types of proteins wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
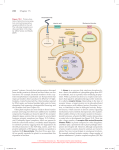
Introduction • Survival of an organism depends on appropriate responses to the environment, both internal and external. • Just as it is important for humans to respond appropriately in their daily lives, cells must respond appropriately to stay alive. • Cells are like tiny computers processing millions of inputs and generating millions of outputs daily. • Signals originate from the general environment or from other cells, local or distant. • Three sequential processes are involved in the cell’s response to a signal. • The signal binds to a receptor protein. • The binding causes a message to be sent to the cell’s cytoplasm and to be amplified there. • The cell changes its activity in response to the signal. • The communication process, called signal transduction, results in a change in the function of the cell. • The signaling pathways are like switches in sophisticated electrical systems. Complex cellular behaviors can result from the interactions of many simple switching systems. • (See Video 15.1.) Signals • Both prokaryotic and eukaryotic cells must process information from their environment and calculate appropriate responses. • Signals may be chemical molecules or physical stimuli like light or heat. • Cells must be set up to interpret signals—not all cells can interpret all signals. • To interpret a signal, a cell must have the appropriate receptor protein. • In multicellular organisms, all cells have the genetic information for all receptor proteins, but due to differential gene expression, different cells have different receptors. Cells receive signals from the physical environment and from other cells • Organisms receive many signals from the environment. Our sense organs allow us to respond to light, odors, tastes, temperature, touch, and sound. Plants respond to light and temperature. Some birds and bacteria orient themselves to Earth’s magnetic poles. • Multicellular organisms’ internal cells are exposed to extracellular fluids and other cells, from which they receive information. • A few of the many types of signals in animal cells are hormones, neurotransmitters, chemical messages from the immune system, CO2, and H+. O2 and changes in osmotic pressure are also signals. • In large animals, signals reach targets via diffusion as autocrine or paracrine signals when the target is close. • Autocrine signals are signals generated by the same cells upon which they act. • Paracrine signals diffuse to and affect nearby cells. • When the target is distant, signals travel by circulation in the blood. (See Figure 15.1.) • Signals are involved in a multitude of other cellular and organismal activities such as cell replacement and cell death, the healing of wounds, the maintenance of nutrient concentrations, and processing information from light and sound. A signal transduction pathway involves a signal, a receptor, transduction, and effects • The entire signaling process, from signal detection to final response, is called a signal transduction pathway. • When scientists discuss cell signaling, they tend to describe it as a series of events. • Generally, many of the events in the pathway are actually happening at the same time. • For example, some signals arrive at the cell while the effects of previous signals are still being processed. • The signaling process of cells can be compared to telephone communication. Just as your voice is converted first to electrical impulses, then to digital information, then to electrical impulses, and then back into sound, cell signals are received, interpreted by cells, and then transformed into appropriate outcomes. • The way in which single-celled organisms respond to signals has much in common with signaling in more complex animals and plants. • A sample prokaryotic signal pathway is shown in Figure 15.2. • In E. coli, EnvZ is a transmembrane protein that acts as the receptor for changes in solute concentration. It extends into the space between the plasma membrane and the porous outer membrane, which forms a complex with the cell wall. • Increases in the ion concentration in the space between the two membranes cause the EnvZ protein to change shape. • The shape change, initiated in the intermembrane space, affects regions on both sides of the membrane. • The EnvZ protein becomes an active enzyme, a protein kinase, due to the shape change. The enzyme activity is found on the region of EnvZ facing the cytoplasm. • Protein kinases are common intermediaries in signal transduction. They add a phosphate to a certain amino acid of the proteins for which they are specific. • The EnvZ protein first catalyzes phosphorylation of one of its own histidine residues, again causing a conformational change. • EnvZ then binds to OmpR, a protein that acts as a responder in this signal transduction pathway; EnvZ transfers its phosphate to OmpR. • The OmpR, in a sense, dephosphorylates the EnvZ, resetting EnvZ back to its original state. • The phosphorylated OmpR binds to the promoter of the ompC gene on E. coli’s DNA, adjacent to the sequence that codes for the protein OmpC. This initiates transcription of the ompC gene. • The protein made by the ompC gene, the OmpC protein, inserts into the outer membrane, preventing solutes from entering and restoring the appropriate osmotic condition. • The steps in this signal transduction pathway occur in many other signal transduction systems in animals and plants: • A receptor changes conformation (shape) upon binding its specific signal molecule. • Conformational change results in kinase activity. • Phosphorylation alters the functioning of a protein. • The signal is amplified. • Transcription factors are activated. • Altered synthesis of specific proteins occurs. • Protein action alters cell activity. Receptors • • A cell responds to only a few of the signals it receives. The type of receptors each cell makes is genetically determined. Receptors have specific binding sites for their signals • A ligand is the signaling molecule that binds the receptor. (See Figure 15.3.) • Receptors change shape when bound. • Ligands are often only signaling molecules, not metabolites. Unlike substrates in their interactions with enzymes, ligands usually are not metabolized into useful products. • Receptors bind ligands according to chemistry’s law of mass action. (See Chapter 2.) • Therefore, binding is reversible, although most ligand–receptor complexes favor binding, so the equilibrium point is far to the right. • The concentration necessary to saturate receptors depends on the affinity of the receptor for the ligand. • Some have high affinity, which means low concentrations can have large effects. • Some receptors bind inhibitors as well as signals. • Inhibitors that bind the receptor’s ligand-binding site are called competitive inhibitors. • Inhibitors that bind at sites other than the ligand-binding site are called noncompetitive inhibitors. There are several types of receptors • The cellular location of receptors depends on the nature of their ligands. • There are two major classes of signaling molecules, based on polarity. • Nonpolar signaling molecules can pass through the plasma membrane. (See Figure 15.4.) • Steroids are examples of nonpolar signaling molecules. • Estrogen, for example, can diffuse easily across the plasma membrane. • Steroids bind receptors that are located in the cytoplasm. • Only cells expressing the genes that code for a receptor of a certain steroid can be affected by that steroid. • There are other signal molecules besides steroids that can pass directly through the membrane. • Large or polar signals cannot cross the membrane. • They must interact with the outside portion of receptors embedded in the cell’s membrane. • Receptors for large or polar signals are generally transmembrane proteins. • Insulin is a protein hormone that interacts with a transmembrane receptor. (See Figure 15.4.) • Three well-studied types of transmembrane receptors in complex eukaryotes are ion channels, protein kinases, and G protein-linked receptors. (See Animated Tutorial 15.1.) • Ion channel receptors: Some ion channel proteins, acting as “gates,” are signal receptors. • Channel proteins can open to let in or out, or close to restrict, certain ions. (See Figure 15.5.) • The signal to open or close the channel can be chemical, light, sound, pressure, or voltage. • An example of a gated ion channel is the acetylcholine receptor. (See Figure 15.5.) • Protein kinase involvement in cell signaling is diverse and common. • Some eukaryotic receptor proteins become kinases when activated. • A phosphate is transferred from ATP to a certain amino acid (residue) in a protein, changing the state of the protein from active to inactive, or vice versa. • Sometimes the protein kinase phosphorylates itself. This is called autophosphorylation. • In eukaryotic cells, the usual target for phosphorylation is tyrosine, serine, or threonine (at the terminal end of these amino acids’ R group). • Insulin receptors are examples of protein kinase receptors. (See Figure 15.6.) • The insulin receptor consists of two subunits, which bind the ligand (insulin) outside the cell, and two subunits, which transmit the signal to the cytoplasm. • Two molecules of insulin (one for each subunit) must bind to the receptor in order to transmit the signal. • After binding insulin on its extracellular surface, the receptor changes its shape. • The shape change exposes a protein kinase active site at the cytoplasmic region of the receptor. • Like the EnvZ receptor in E. coli, the insulin receptor self-phosphorylates. • The insulin receptors can then target and phosphorylate other cytoplasmic proteins called insulin response substrates. • These proteins then initiate other cellular activities, including the insertion of glucose transporters into the cell membrane. • Insulin and its receptors are part of the family of enzyme-linked transmembrane receptor systems. • Another type of signal receptor, the “seven-spanning G protein-linked receptor,” exists at the beginning of a modular-type system of information transfer. (See Figure 15.7.) This system consists of at least three units: a receptor that spans the plasma membrane, a membrane protein called a G protein, and an effector protein. • What is a G protein? • G proteins have a binding site for the G protein-linked receptor and for a nucleotide called GDP/GTP. • GTP and GDP, like ATP and ADP, are ribonucleotides. Whereas ATP is a common energy molecule and also a building block for RNA, GTP is used in some signaling pathways and as a building block for RNA. • G proteins have GTPase activity as well. This means that G proteins can hydrolyze the terminal phosphate from GTP to form GDP. • G proteins are active when they are bound to GTP and inactive when they are bound to GDP. • G proteins are also important constituents of the cell’s cytoskeleton. • As with many other receptor systems, a signal from outside the cell binds the G protein-linked receptor and changes its shape both outside and inside the cell. • The changed shape in the internal domain of the receptor causes it to bind a membrane-associated G protein. • When the receptor and the G protein bind, a subunit of the G protein releases its GDP and acquires a GTP. • The GTP activates the subunit of the G protein, which separates from the rest. • The GTP-bearing subunit moves along the plane of the internal plasma membrane until it encounters and activates an effector molecule. • After the interaction, the G protein hydrolyzes its GTP, thereby switching its own activity back to the initial off state. • To complete the cycle, this G protein subunit must find another G protein receptor, minus the subunit, and associate with it. • When a signal causes the subunit to release its GDP, the cycle begins again. • G proteins can either activate or inhibit effectors. Epinephrine (the “fight or flight” hormone) illustrates both possibilities. • In the heart, a G protein-linked receptor system activates an enzyme. • The heart is set up to respond to epinephrine as a signal. • G protein-linked receptors in heart muscle bind epinephrine. • The associated G protein gets activated in response to the change in receptor shape. • The G protein subunit swaps GDP for GTP and diffuses along the plasma membrane. • It activates another enzyme associated with the cytosolic face, which makes a small molecule called cAMP (cyclic adenosine monophosphate). • cAMP, in turn, has a wide range of effects on the cell, including glucose mobilization and muscle contraction. • In the smooth muscle cells surrounding blood vessels lining the digestive tract, a G protein-linked receptor system inhibits an enzyme. • The system begins the same way as in the heart: Epinephrine activates the receptor, which binds a G protein, which activates a subunit, which diffuses along the inside of the plasma membrane. • In this case, however, when the G protein subunit encounters its target enzyme, it inhibits the enzyme instead of activating it. As a result, the muscles relax, the blood vessel dilates, and the flow of nutrients away from the stomach to the rest of the body increases. • This example demonstrates that the same signal and initial signaling mechanism can have different consequences in different cells. The response depends on how the cell is set up to respond. • Not all signal receptors act at the plasma membrane. Some signaling molecules get through the membrane and into the cytoplasm, where they react with cytoplasmic receptor proteins. (See Figure 15.8.) • Steroid hormones are an example of such signal molecules. • Some cells have cytoplasmic receptors for certain steroids such as cortisol. • The intracellular receptor binds to the steroid, changing the shape of the receptor. • This causes it or some event-related component to bind DNA and influence gene expression as a transcription factor. Signal Transduction • Transducers convert signals from one form to another. An example of transducing is the conversion of electrical impulses to sound in common loudspeakers. • Direct transduction results from the action of the receptor itself on effector proteins; it occurs at the plasma membrane. • Indirect transduction, which is more common, uses a second messenger to mediate the interaction between receptor binding and cellular reaction. • These can cause signals to branch, converge, or amplify. • Neither direct nor indirect transduction is a single event. In both cases, the signal initiates a series of protein– to–protein events that eventually lead to a final response. Protein kinase cascades amplify a response to receptor binding • A cascade is a connected series (e.g., a series of waterfalls, or amplifiers) for increasing an output. • A protein kinase cascade is direct signal transduction that catalyzes the phosphorylation of target proteins. • Details of a certain protein kinase cascade were discovered from the investigation of Ras protein inhibition as treatment for bladder cancer. (See Figure 15.9.) • In cancer cells the Ras protein is always bound with GTP because the GTPase activity is inoperative. • Therefore, the Ras protein causes constant phosphorylation and continuous cell division. • Ras is part of a protein kinase cascade that influences cell division. The pathway is called a cascade because each kinase phosphorylates the next. • Protein kinase receptors stimulate the protein kinase cascade right at the plasma membrane. • The unbound receptors for growth factors exist in the plasma membrane as polypeptide chains (subunits). • When the growth factor signal binds to a subunit, it associates with another subunit to form a dimer, which changes its shape to expose a protein kinase active site. This activates several other protein kinases in turn. • The final phosphorylated protein—MAP kinase—moves into the nucleus and phosphorylates target proteins necessary for cell division. • There are at least three advantages to having many kinase steps in signal transduction. • Each activated protein kinase can phosphorylate many target proteins, so amplification of the signal occurs at each step, and a small input signal becomes a large output signal. • Information from a signal at the cell membrane is transferred to the nucleus. • Having a variety of steps affecting different target proteins allows for a variety of responses by different cells to the same signal. Cyclic AMP is a common second messenger • Indirect transduction is more common than direct transduction. • Scientists investigating the effects of epinephrine on liver cells discovered cyclic AMP (cAMP) as a second messenger. • Studying the sequence of events by breaking apart liver cells, they learned that to activate an enzyme called phosphorylase, both plasma membrane and signal hormone (epinephrine) were necessary. • They incubated broken plasma membranes with epinephrine, then removed the plasma membranes and added the resulting solution to liver cell cytoplasm that contained inactivated phosphorylase enzyme. • The phosphorylase enzyme became activated. • Therefore, the epinephrine and plasma membrane must have generated a soluble second messenger. • This was found to be cAMP. (See Figure 15.10.) • The cAMP molecule is a small cyclic nucleotide generated from ATP. • The enzyme adenylyl cyclase produces cAMP using ATP as a substrate. Adenylyl cyclase is activated to produce cAMP by an activated G protein subunit. • cAMP controls many different cellular activities by amplifying signals which then activate many enzyme targets. • Like other second messengers, cAMP is not an enzyme. Second messengers act as cofactors or allosteric regulators of target proteins. • cAMP has two major kinds of targets: ion channels and protein kinases. • Some cells are set up to respond to cAMP uniquely. • Certain cell types, like the follicular cells of the ovary, have unique receptors, which when bound by their ligand cause a rise in cytosolic cAMP. • Downstream, this influences steroid synthesis. Two second messengers are derived from lipids • Phospholipids, in addition to their roles as structural components of the plasma membrane, are involved in signal transduction. • Certain phospholipids get hydrolyzed by an enzyme called phospholipase C. (See Figure 15.11.) • What activates the phospholipase C? • A signal at the outside of the cell attaches to the ligand-binding site of the receptor protein, a G proteinassociated receptor protein. • The G protein subunit swaps GDP for GTP and becomes active. • This particular G protein subunit is specific for phospholipase C. It slides around the internal face of the plasma membrane until it encounters a phospholipase C molecule. • Active phospholipase C hydrolyzes the phospholipid called phosphatidyl inositol-bisphosphate (PIP2) into inositol triphosphate (IP3) and diacylglycerol (the two fatty acid tails of the lipid, abbreviated DAG). • The two parts generated by the hydrolysis of the phospholipid PIP2 each become second messengers, with IP3 moving into the cytoplasm and DAG remaining in the membrane. • These second messengers trigger many cellular events. • One of DAG’s targets is a membrane-bound enzyme, a kinase called protein kinase C (PKC). • IP3 diffuses throughout the cytoplasm until it contacts certain Ca2+ channels found in the endoplasmic reticulum (ER). • Calcium levels are normally low in the cytosol and high in the ER. • When IP3 opens the Ca2+ channels in the ER, Ca2+ diffuses down the concentration gradient into the cytoplasm. • The Ca2+ ion can also stimulate its own release from intracellular stores. • So far we have seen that one signaling event outside the cell diverges into two, with the generation of two different second messenger molecules. • These two signaling paths converge at protein kinase C. • To be activated, protein kinase C needs both DAG and Ca2+; the Ca2+ is provided when IP3 opens the Ca2+ channels in the ER. • A number of other events that are not convergent also occur. Calcium ions are involved in many signal transduction pathways • Calcium ions are also second messengers. • Ca2+ concentration in the cytoplasm is usually only about 0.1 . • The concentration is kept low via active transport, both out of the cell and into the ER. • Unlike cAMP, Ca2+ cannot be manufactured in the cell; it must be imported. • Many different signals cause Ca2+ channels to open, including IP 3. • The entry of a sperm into an egg cell also causes Ca2+ channels to open. (See Figure 15.12.) • Another signal that opens Ca2+ channels is electrical depolarization in muscle cells. • Once a signal triggers Ca2+ channels to open, Ca2+ concentration rapidly rises to approximately 100 times the resting concentration. • The calcium ions then affect the activities of cellular proteins, including protein kinase C. • Ca2+ also binds to Ca2+ channel proteins, triggering additional releases of Ca2+. • This can be described as a positive feedback loop. • Calcium ions bind to a calcium-binding protein called calmodulin, which can activate certain proteins. • Calmodulin has four calcium binding sites for Ca2+. • At low Ca2+ concentrations, the chance that all four Ca2+ binding sites are occupied at once is low. • At high Ca2+ concentrations, all four binding sites are filled, and some of the calmodulin molecules become active via a change in conformation. • Calmodulin then can activate target molecules. • One target in muscle cells is a myosin-specific protein kinase needed for initiating muscle contraction. • (See Video 15.2.) Nitric oxide is a gas that can act as a second messenger • The gas nitric oxide (NO) was found to be a second messenger by scientists studying the effects of acetylcholine. • Acetylcholine causes the relaxation of smooth muscles of the blood vessels. (See Figure 15.13.) • The sequence of events understood then was as follows: • Acetylcholine molecules bind G protein-linked receptors. • The activated G protein subunits trigger phospholipase C to hydrolyze lipid. • Acetylcholine stimulates the IP3 pathway to produce an influx of Ca2+, which leads to an increase in the level of another second messenger, cGMP. • The target is a tissue-specific protein kinase, which via phosphorylation causes muscle relaxation. • This series of events was determined from blood vessels in intact animals. • In studies by Furchgott, however, isolated strips of arterial tissue failed to respond, whereas tubular sections of artery did. Only the latter tissue had the endothelium (the inner layer or cells lining blood vessels) intact. • After difficult work, it was discovered that NO (which previously had been thought of only as an air pollutant!) is needed. NO is produced by the endothelial cells, which are close to smooth muscle cells. • Acetylcholine causes increased Ca2+ levels in these endothelial cells. • The elevated Ca2+ levels cause, among other effects, the activation of NO synthase (the enzyme that makes NO a very unstable molecule). • NO diffuses rapidly from the endothelial cells to the nearby smooth muscle cells. • In the smooth muscle cells of blood vessels, NO activates the enzyme guanylyl cyclase, which stimulates the formation of cGMP. • NO as a second messenger explains the action of certain drugs. • Nitroglycerin, which had long been used to treat angina, is now understood as a releaser of NO, resulting in relaxation of the blood vessels and increased blood flow to the heart. • Certain newer drugs that promote penile erection also are NO synthesis activators. Signal transduction is highly regulated • Cells must regulate the activity of a transducer in order to respond to stimuli and then return quickly to a restored state. • NO molecules are unstable and break down quickly, so NO is regulated by how much of it is made. • Ca2+ concentrations, on the other hand, are restored to normal by mechanisms such as membrane pumps and ion channels that close the Ca2+ channels and pump Ca2+ out of the cytosol. • Protein kinase cascades are interrupted by protein phosphatases that remove the added phosphates, deactivating the kinases. • GTPases deactivate G proteins by converting GTP to GDP. • Both cAMP and cGMP are converted to AMP and GMP by their respective phosphodiesterases. Signal Effects: Changes in Cell Function • Effects of signaling are manifest in the opening of membrane channels, changes in enzyme activity, and differences in gene transcription. Ion channels are opened • Sensory nerve cells of the sense organs are stimulated through the opening of ion channels. • Some mammals have as many as 1,000 genes that code for odorant receptors—the largest gene family known. • Considering current estimates for the total number of human genes (50,000–100,000), the possibility that of all these genes is dedicated to the detection of odor is rather astounding! • Each of the thousands of nerve cells in the nose expresses just one of these receptors. • When an odorant molecule binds to its receptor, a G protein becomes activated. (See Figure 15.14.) • The activated G protein subunit causes adenylyl cyclase to make the second messenger, cAMP. • The cAMP binds to ion channels, causing them to let in Na+. • The change in Na+ ion concentration stimulates the neuron to send a signal to the brain, which perceives the signal as a scent. • Some odor molecules bind to more than one type of odorant receptor. This provides a means for detecting and distinguishing among enormous numbers of different odors. Enzyme activities are changed • The effects of epinephrine on liver cells are an example of signal transduction resulting in altered enzyme activity. (See Figure 15.15.) • The binding of epinephrine to a G protein-linked receptor results in synthesis of cAMP, which in turn initiates a series of kinase reactions. • Included in the effects of this signal transduction pathway is the altering of two enzymes: • Glycogen synthase is deactivated by phosphorylation. When active, this enzyme catalyzes the joining of glucose molecules into glycogen for storage. Deactivation of this enzyme therefore slows storage of glucose. • Glycogen phosphorylase is activated. This enzyme catalyzes the release of glucose molecules from glycogen. Different genes are transcribed • Plasma membrane receptors are involved in initiating a broad range of gene expression responses. • Ras signaling pathways end in the nucleus. (See Figure 15.10.) • The final protein kinase phosphorylates a DNA binding protein called AP-1. • This activated AP-1 protein now can bind to DNA and influence gene transcription. • Finally, a number of genes involved with cell division are transcribed. • Lipid-soluble steroid hormones bind to receptors in the cytoplasm, which then influence gene transcription. • In plants, light acts as a signal to begin chloroplast formation. • Bright light activates phytochrome, which then binds to cytoplasmic regulatory proteins found in the cytoplasm. • These can then move to the nucleus, bind DNA, and influence gene transcription, leading to the synthesis of important chloroplast proteins. Direct Intercellular Communication • Some cells send signals directly from their interior to the interior of adjacent cells. • This transfer occurs by way of specialized structures called gap junctions in animal cells, and plasmodesmata in plant cells. Animal cells communicate by gap junctions • Gap junctions permit metabolic cooperation among linked animal cells. (See Figure 15.16.) • Gap junctions are complexes of proteins that generate channels, called connexons, in adjacent cell membranes. • Two connexons, one from each cell, form the channel. There can be hundreds of channels between a cell and its neighbor. • Each connexon is made up of six small snap-together subunits called connexins. • The channels can be gated. • The channel is small relative to macromolecules, but large enough for small signal molecules and ions to pass. • Gap junctions ensure the sharing of ATP, metabolic intermediates, amino acids, and coenzymes. Signal molecules such as hormones and second messengers such as cAMP and PIP2 also can move through gap junctions. • Ca2+ molecules can also pass through the gap junction. • In the developing mammalian egg, for example, the surrounding granulosa cells form gap junctional complexes with the egg. • Some evidence exists that cAMP levels maintained by the granulosa control the state of development of the egg. Plant cells communicate by plasmodesmata • In plant cells, communication directly from the cytoplasm of one cell to another is through plasmodesmata. (See Figure 15.17.) • Plasmodesmata are membrane-lined bridges spanning the thick cell walls between adjacent cells. • Generation of plasmodesmata involves fusion of adjacent plasma membranes to make the channnel. • The channel is about three times as large as the ones in gap junctions, but a tube called the desmotubule fills most of the channel. Therefore, the space for molecular traffic is usually about the same as in gap junctions (approximately 1.5 nm) and generally only small molecules move through. • Plasmodesmata are important to C4 plants, helping them to move fixed carbon between mesophyll and bundle sheath cells. • Plasmodesmata pore size can be regulated. Some very large proteins, as well as viruses and their RNA, have been found to move through temporarily enlarged channels.