* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 5)qualitative_tests_of_proteins
Evolution of metal ions in biological systems wikipedia , lookup
Peptide synthesis wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Paracrine signalling wikipedia , lookup
Point mutation wikipedia , lookup
Gene expression wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Signal transduction wikipedia , lookup
Biosynthesis wikipedia , lookup
Expression vector wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Magnesium transporter wikipedia , lookup
Genetic code wikipedia , lookup
Metalloprotein wikipedia , lookup
Interactome wikipedia , lookup
Protein purification wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Western blot wikipedia , lookup
Biochemistry wikipedia , lookup
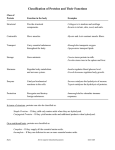
PROTEINS Introduction: - Protein (from the Greek protas meaning "of primary importance") is a complex, highmolecular-weight organic compound that consists of amino acids joined by peptide bonds. - Proteins are natural polymer molecules consisting of amino acid units. The number of amino acids in proteins may range from two to several thousand. - Proteins are probably the most important class of biochemical molecules, although of course lipids and carbohydrates are also essential for life. Proteins are the basis for the major structural components of animal and human tissue. - Proteins are essential to the structure and function of all living cells and viruses. Many proteins are enzymes or subunits of enzymes, catalyzing chemical reactions. Other proteins play structural or mechanical roles, such as those that form the struts and joints of the cytoskeleton, serving as biological scaffolds for the mechanical integrity and tissue signaling functions. - Proteins can be hydrolyzed by acids, bases or specific enzymes. Primary Protein Structure: - Proteins are biopolymers built from 20 different L-alpha-amino acids. - The two ends of the amino acid chain are referred to as the carboxy terminus (Cterminus) and the amino terminus (N-terminus) based on the nature of the free group on each extremity. - Biochemists refer to four distinct aspects of a protein's structure: Page 1 Organized by: Sharifa Al-Ghamdi& Huda Al-Shaibi 1- Primary structure: the amino acid sequence. In order to function properly, peptides and proteins must have the correct sequence of amino acids. 2- Secondary structure: is the specific geometric shape caused by intramolecular and intermolecular hydrogen bonding of amide groups. Some combinations of amino acids will tend to form: Alpha Helix: In the alpha helix, the polypeptide chain is coiled tightly in the fashion of a spring. The "backbone" of the peptide forms the inner part of the coil while the side chains extend outward from the coil. The helix is stabilized by hydrogen bonds between the >N-H of one amino acid and the >C=O on the 4th amino acid away from it. Beta Pleated Sheet: In this structure, individual protein chains are aligned side-by-side with every other protein chain aligned in an opposite direction. The protein chains are held together by intermolecular hydrogen bonding, that is hydrogen bonding between amide groups of two separate chains. This intermolecular hydrogen bonding in the beta-pleated sheet is in contrast to the intramolecular hydrogen bonding in the alpha-helix. Page 2 Organized by: Sharifa Al-Ghamdi& Huda Al-Shaibi 3- Tertiary structure: is the entire three-dimensional shape of the protein. This shape is determined by the sequence of amino acids. The overall shape of a single protein molecule primarily formed by hydrophobic interactions, but hydrogen bonds, ionic interactions, and disulfide bonds are usually involved too. 4- Quaternary structure: the shape or structure that results from the union of more than one protein molecule, usually called protein subunits in this context, which function as part of the larger assembly or protein complex. Page 3 Organized by: Sharifa Al-Ghamdi& Huda Al-Shaibi Denaturation of Proteins: - Denaturation is the disruption of secondary, tertiary and quaternary structure of proteins leading to loss of their biological activity. - Proteins denature when they lose their three-dimensional structure - their chemical conformation and thus their characteristic folded structure. Proteins may be denatured at the secondary, tertiary and quaternary structural levels, but not at the primary structural level. - Denaturation may be caused by: 1- Physical factors such as heating. 2- Chemical factors such as strong acid or base. Denaturated proteins are characterized by: 1-Loss of function: Most biological proteins lose their biological function when denatured, for example, enzymes lose their catalytic activity. 2- They become less soluble. As a result, they are easily precipitated. Page 4 Organized by: Sharifa Al-Ghamdi& Huda Al-Shaibi 3- Reversibility and irreversibility: In many proteins (unlike egg whites), denaturation is reversible (the proteins can regain their native state when the denaturing influence is removed). Page 5 Organized by: Sharifa Al-Ghamdi& Huda Al-Shaibi Qualitative Tests for Proteins 1- Biuret Test: - It is the general test for all proteins. - Biuret reagent is dilute CuSO4 in strong alkaline medium. - Alkaline CuSO4 reacts with all compounds containing 2 or more peptide bonds to give a blue-violet color. Method: 1 ml of biuret reagent + 1 ml of protein ……mix well>>>> blue-violet color. 2- Denaturation by heat and extreme pH: - Extreme heating and pH (conc. acids) denature proteins leading to precipitation of proteins. Method: 3ml Protein >>>>>>BWB-10min >>>>>> ppt of protein. 3ml Protein >>>>>> drops conc.HCL >>>>>> ppt of protein. Page 6 Organized by: Sharifa Al-Ghamdi& Huda Al-Shaibi 3- Precipitation of proteins by heavy metals: - Proteins are precipitated in alkaline medium with heavy metals due to the direct union of cation (Cu++, Ag+, Ba++, Pb++) with anionic groups of proteins, which are formed in basic medium. - At alkaline pH 7 and above, proteins are usually negatively charged so the addition of positively charged ions will neutralize this charge and the proteins come out of solution (i.e. heavy metals combine with proteins forming insoluble metalloproteine). Method: Few drops of heavy metals + 2ml protein + few drops 10% NaOH>>>>ppt 4- Precipitation of proteins by acidic reagent: - Proteins are precipitated in acidic medium with some reagents such as TCA, picric acid and tannic acid due to the direct union of the anionic group with the cationic groups of the proteins, which are formed in acidic medium. - These compounds carry large negative charges which neutralize the positively charged protein to form insoluble salt complex with protein. - The acidic reagents are therefore most effective at acidic medium where proteins are positively charged. Method: Few drops of acidic reagent + 2ml protein >>>slowly add dilute NaOH and observe the result as the pH increase. 3- Detection of Amino acids contents of Protein: Carry on all the experiments you have done in amino acids lab on proteins to detect the amino acid content of each protein. References: www.chemtopics.com www.wikipedia.org Page 7 Organized by: Sharifa Al-Ghamdi& Huda Al-Shaibi RESULTS & LAB REPORT - Present your results in a good and full lab report. Page 8 Organized by: Sharifa Al-Ghamdi& Huda Al-Shaibi