* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Supplementary information for Ronshaugen, McGinnis
Promoter (genetics) wikipedia , lookup
List of types of proteins wikipedia , lookup
Secreted frizzled-related protein 1 wikipedia , lookup
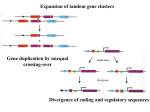
Molecular evolution wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Magnesium transporter wikipedia , lookup
Gene regulatory network wikipedia , lookup
Immunoprecipitation wikipedia , lookup
Protein moonlighting wikipedia , lookup
Point mutation wikipedia , lookup
Protein adsorption wikipedia , lookup
Gene expression profiling wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Gene expression wikipedia , lookup
Expression vector wikipedia , lookup
Supplementary information for Ronshaugen, McGinnis, & McGinnis, “Hox protein mutation and macroevolution of the insect body plan. Nature M10588 Cloning arthropod Hox genes. Full length Artemia franciscana Ubx and Antp protein coding sequence was obtained from mRNA extracted from Artemia nauplii collected 5 days after hatching. A fragment of the Artemia Ubx and Antp homeodomain was obtained using primers and cycling conditions similar to Pendleton et al.1 except in primers, where inosine was replaced with a fourfold degenerate site, and in the thermocycle, where the annealing temperature was 400C. These homeodomain fragments were used to design primers for 3’ and 5’ RACE (rapid amplification of cDNA ends) using the Marathon cDNA amplification system (Clontech). Primers were constructed in the 5’ and 3’ UTR of the RACE products and used to amplify full-length cDNA’s from multiple independent poly-T primed reverse transcriptase (RT) reactions. The final protein sequences were derived from a consensus sequence obtained from the assembly of RACE products derived from multiple independent RT reactions and verified against genomic sequence. Arthropod Ubx C-terminal sequences shown in Figure 4 were obtained in a similar manner. Antibody stainings and in situ hybridizations. Epitope-tagged Hox genes were incubated with either a rat monoclonal antibody (Roche) directed against the hemagglutinin antigen (anti-HA) at a 1:50 dilution, or the monoclonal antibody FP6.87 2 directed against either Ubx or AbdA at a 1:3 dilution. Beta-galactosidase protein from reporter genes was detected with rabbit polyclonal antiserum at 1:100 (Cappel Research Products). Detection of anti-HA and beta-galactosidase was accomplished with the following fluorescent secondary antibodies; Cy3 conjugated donkey anti-rabbit (Jackson Labs); Cy3 conjugated donkey anti-rat (Jackson Labs), fluorescein conjugated rabbit antirat (Vector Labs), fluorescein conjugated donkey anti-mouse (Jackson Labs). Visualization of FP6.872 was accomplished with a biotinylated goat anti-mouse secondary (Jackson Labs), Vectastin elite ABC kit and detected with the TSA florescein system (NEN). In situ hybridizations to detect transcripts were performed using a variation of the protocol described by Tautz and Pfeifle 3. Digoxigenin-labeled antisense probes for Dll were made from a 1.2 EcoR1 cDNA fragment 4 and Antp P1 and P2 specific probes were made from plasmids described in Bermingham et al. 5. Normalization of ectopic protein levels. The FP6.87 antibody was shown to have identical affinity for Drosophila and Artemia Ubx on a western blot in which equivalent amounts of in vitro translated protein was loaded (data not shown). This antibody was then used to establish the average level of endogenous Ubx in the ventral first abdominal segment for stage 11-12 embryos reared at 25 degrees. This average was determined by randomly sampling the mean luminosity in a 1 second (underexposed) image for a 50X50 pixel region in the middle of the A1 segment in appropriately staged animals. In parallel, multiple independent lines of Artemia Ubx HA and Drosophila Ubx HA were crossed to the arm-Gal4 driver and reared at 25 and 29 degrees. These embryos were assayed for expression in the ventral second thoracic segment. Specific lines of Artemia Ubx HA and Drosophila Ubx HA were chosen that had levels of ectopic protein nearest to the average determined for the wild type A1 segments. Identically treated embryos were then stained with a rat monoclonal anti-HA antibody and the mean luminosity with this antibody in the T2 segment was determined. All other transgenic lines were similarly assayed using the anti-HA antibody. These were selected based on similarity to the mean luminosity with the anti-HA antibody in Artemia Ubx HA embryos. The maximum variation in expression levels allowed was ~30% which was estimated to be less than the standard error for the average expression levels within a particular line. The level of ectopic protein detected in the second thoracic segment at stage 11-12 relative to Artemia Ubx are as follows: Drosophila Ubx ; 0.9, Dros281Art; 0.8 , Dros356Art; 0.7, Art C-term; 0.7, Dros QA; 0.7, Art250Dros QA; 0.8, Artemia Ubx 5S/T to A; 0.7, Artemia Ubx 6 S/T to A; 0.7. Accession numbers for the amino acid sequences shown in Figure 4. Fruit fly sequences are from Drosophila melanogaster (Ubx - P02834; Antp - CAA27417); mosquito Ubx, Anopheles gambiae (AAC31942); moth Ubx, Manduca sexta (AAB39544); beetle, Tribolium castaneum (Antp - AAK96031, Ubx - AAD38009), springtail Ubx, Folsomia candida (AF435789), brine shrimp, Artemia franciscana (Antp - AF435786, Ubx - AF435787), copepod Ubx, Tigriopus californicus (AF435788), isopod from Porcellio scaber (Antp - AAK13077, Ubx - AF435791), centipede, Ethmostigmus ribripes (Antp - AAB91389, Ubx - AAB91393), millipede Ubx, Archispirostreptus (AF435790), spider Ubx, Cupiennius salei (CAA07501). 1. Pendleton, J. W., Nagai B. K., Murtha M. T. & Ruddle F. H. Expansion of the Hox gene family and the evolution of chordates. Proceedings of the National Academy of Science. 90, 6300-6304 (1993). 2. Kelsh, R. R. O. J. W., White, R. A. H. & Akam, M. Homeotic gene expression in the locust Schistocerca: An antibody that detects conserved epitopes in ultrabithorax and abdominal-A proteins. Dev. Genet. 15, 19-31 (1994). 3. Tautz, D. & Pfeifle, C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma 98, 81-85 (1989). 4. Cohen, S. M., Brönner, G., Küttner, F., Jürgens, G. & Jäckle, H. Distal-less encodes a homeodomain protein required for limb development in Drosophila. Nature 338, 432-434 (1989). 5. Bermingham, J. R., Martinez-Arias, A., Petitt, M. G. & Scott, M. P. Different patterns of transcription from the two Antennapedia promoters during Drosophila embryogenesis. Development 109, 553-566 (1990).