NMR - University of Puget Sound
... Sidechain location vs. polarity -Nonpolar residues in interior of protein (hydrophobic effect promotes this, as well as efficient packing of those sidechains) -Charged polar residues on protein surface (immersing charge in anhydrous interior is energetically unfavorable) -Uncharged polar groups occu ...
... Sidechain location vs. polarity -Nonpolar residues in interior of protein (hydrophobic effect promotes this, as well as efficient packing of those sidechains) -Charged polar residues on protein surface (immersing charge in anhydrous interior is energetically unfavorable) -Uncharged polar groups occu ...
Organic Chemistry
... Globular vs. Fibrous Proteins? Functions of Structural Proteins? Functions of Globular Proteins? Structure/Function Relationships? ...
... Globular vs. Fibrous Proteins? Functions of Structural Proteins? Functions of Globular Proteins? Structure/Function Relationships? ...
Metal Regulation and Signalling - Zn Proteins
... bioinorganic research moves beyond metalloenzymes to more subtle roles for metals: structural roles ...
... bioinorganic research moves beyond metalloenzymes to more subtle roles for metals: structural roles ...
Chapters 2 - 5 Exam Prep: What to Know
... What is a buffer? How does a buffer help to maintain pH? Know the pH of acids and bases. Vitalism vs mechanism. Go over/review the Miller/Urey experiment; reactants, products, importance. Isomers and enantiomers. Thalidomide and L-dopa. Know all the biological molecules and characteristics of each. ...
... What is a buffer? How does a buffer help to maintain pH? Know the pH of acids and bases. Vitalism vs mechanism. Go over/review the Miller/Urey experiment; reactants, products, importance. Isomers and enantiomers. Thalidomide and L-dopa. Know all the biological molecules and characteristics of each. ...
Macromolecule Scramble
... the association of the polypeptide chains some proteins contain more than one polypeptide chain Each polypeptide chain in the protein is called a subunit Two or more subunits come together for a specific ...
... the association of the polypeptide chains some proteins contain more than one polypeptide chain Each polypeptide chain in the protein is called a subunit Two or more subunits come together for a specific ...
Proteins
... • the haem group is not made of AA, but is an integral part of the protein – prosthetic grp. • Each haem group contains an ion of iron ...
... • the haem group is not made of AA, but is an integral part of the protein – prosthetic grp. • Each haem group contains an ion of iron ...
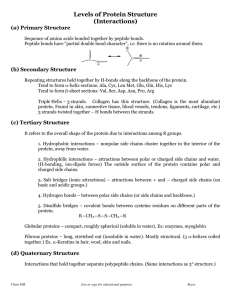
Proteins have a higher order of folding known as tertiary structure
... If two cysteine residues are near to each other, they can react to form a covalent bond known as a disulfide bridge. ...
... If two cysteine residues are near to each other, they can react to form a covalent bond known as a disulfide bridge. ...
more details
... Abstract: Standard models of protein evolution generally assume each location evolves independently, although it is well appreciated that substitution rates in a protein are influenced by changes in the amino acids at other locations. Generating more accurate but computationally tractable models of ...
... Abstract: Standard models of protein evolution generally assume each location evolves independently, although it is well appreciated that substitution rates in a protein are influenced by changes in the amino acids at other locations. Generating more accurate but computationally tractable models of ...
Protein Origami
... makes fireflies glow, and a lot more. Proteins also aid viruses in invading cells. AIDS can be tied to proteins that break through cellular defenses and replicate the HIV virus. Cancer is linked to damage in proteins that inhibit uncontrolled cell growth. Alzheimer’s disease is believed to be relate ...
... makes fireflies glow, and a lot more. Proteins also aid viruses in invading cells. AIDS can be tied to proteins that break through cellular defenses and replicate the HIV virus. Cancer is linked to damage in proteins that inhibit uncontrolled cell growth. Alzheimer’s disease is believed to be relate ...
103 Lecture Ch20b
... • -keratins are fibrous proteins found in feathers and scales that are made up mostly of -pleated sheets ...
... • -keratins are fibrous proteins found in feathers and scales that are made up mostly of -pleated sheets ...
Chapter 5: Biological Molecules Molecules of Life • All life made up
... o Inherited blood disorder o Single amino acid change in protein hemoglobin Amino Acid 6 is Valine instead of Glutamic Acid Alters shape & function Protein Structure o Physical & chemical conditions affect structure, along w/ primary structure Changes in pH, salt, temp, or other environmenta ...
... o Inherited blood disorder o Single amino acid change in protein hemoglobin Amino Acid 6 is Valine instead of Glutamic Acid Alters shape & function Protein Structure o Physical & chemical conditions affect structure, along w/ primary structure Changes in pH, salt, temp, or other environmenta ...
Protein Structure 2 - Interactions - Hydrolysis
... Tend to form α-helix sections: Ala, Cys, Leu Met, Glu, Gln, His, Lys Tend to form β-sheet sections: Val, Ser, Asp, Asn, Pro, Arg Triple Helix – 3 strands. Collagen has this structure. (Collagen is the most abundant protein. Found in skin, connective tissue, blood vessels, tendons, ligaments, cartila ...
... Tend to form α-helix sections: Ala, Cys, Leu Met, Glu, Gln, His, Lys Tend to form β-sheet sections: Val, Ser, Asp, Asn, Pro, Arg Triple Helix – 3 strands. Collagen has this structure. (Collagen is the most abundant protein. Found in skin, connective tissue, blood vessels, tendons, ligaments, cartila ...
R032 Publication Only Basic Science: Biofilm Key proteins of
... metabolism of amino acids, nuclear proteins and protein translation and processing of the RNA. A protein that highlights in proteome analysis is DASH complex subunit that is characterized as a structural constituent of cytoskeleton and was about ten times more expressed in H. capsulatum biofilm. Fur ...
... metabolism of amino acids, nuclear proteins and protein translation and processing of the RNA. A protein that highlights in proteome analysis is DASH complex subunit that is characterized as a structural constituent of cytoskeleton and was about ten times more expressed in H. capsulatum biofilm. Fur ...
FROM TRAIT TO PROTEIN - CLASSROOM
... Part I Proteins are large, complex macromolecules that play critical roles in the body. Proteins are made up of hundreds or thousands of smaller units called amino acids, which are attached to one another in long chains. There are 20 different types of amino acids that can be combined to make a prot ...
... Part I Proteins are large, complex macromolecules that play critical roles in the body. Proteins are made up of hundreds or thousands of smaller units called amino acids, which are attached to one another in long chains. There are 20 different types of amino acids that can be combined to make a prot ...
REVIEW Protein Synthesis with Analogies
... chance by using e-mail or fax to send his plans to the factory. They might be stolen by industrial spies! Donald knows his loyal brother would do anything for him, so he asks him to be a messenger and carry the plans to the factory. At the factory, the assembly line is set up and factory workers bri ...
... chance by using e-mail or fax to send his plans to the factory. They might be stolen by industrial spies! Donald knows his loyal brother would do anything for him, so he asks him to be a messenger and carry the plans to the factory. At the factory, the assembly line is set up and factory workers bri ...
bio98a_l04
... (1) Proteins often become less soluble when the ionic strength is increased to very high levels. Precipitation points vary by protein, so purification can be achieved. (2) Precipitates can be redissolved in a small volume, so concentration can be achieved. (3) Dialyze the redissolved proteins agains ...
... (1) Proteins often become less soluble when the ionic strength is increased to very high levels. Precipitation points vary by protein, so purification can be achieved. (2) Precipitates can be redissolved in a small volume, so concentration can be achieved. (3) Dialyze the redissolved proteins agains ...
CH 460 Dr. Muccio What are the 4 levels of protein structure and
... What are the 4 levels of protein structure and describe each. Do proteins with similar structures usually have similar functions? Primary - amino acid sequence Secondary – helices, sheets, turns, loops Tertiary – 3d folding Quaternary – organization of 3d subunits Yes usually (not always), similar s ...
... What are the 4 levels of protein structure and describe each. Do proteins with similar structures usually have similar functions? Primary - amino acid sequence Secondary – helices, sheets, turns, loops Tertiary – 3d folding Quaternary – organization of 3d subunits Yes usually (not always), similar s ...
PROTEIN PROTEIN: Amino Acids PROTEIN: Complete Proteins
... PROTEIN: Incomplete Proteins Incomplete proteins contain some, but not all, of the amino acids. Incomplete proteins are from other plant sources Examples Include: grains, dried beans, nuts and seeds. Incomplete proteins can be combined to create a complementary protein. ...
... PROTEIN: Incomplete Proteins Incomplete proteins contain some, but not all, of the amino acids. Incomplete proteins are from other plant sources Examples Include: grains, dried beans, nuts and seeds. Incomplete proteins can be combined to create a complementary protein. ...
Key concepts_Protein processing and modification
... complexes called translocons. A number of different mechanisms are employed in bacteria and eukaryotes. In particular, proteins can be translocated either directly from the ribosome, in cotranslational translocation, or from the cytoplasm, in post-translational translocation. The vast majority of pr ...
... complexes called translocons. A number of different mechanisms are employed in bacteria and eukaryotes. In particular, proteins can be translocated either directly from the ribosome, in cotranslational translocation, or from the cytoplasm, in post-translational translocation. The vast majority of pr ...
PLANT PROTEINS FOR THE FUTURE-English
... pigeon pea, etc. are currently the most important legumes for human consumption and animal feed. Amaranth and quinoa are considered “pseudocereals” and are also good sources of proteins. Amaranth seeds contain lysine, an essential amino acid, limited in other grains or plant sources but are limited ...
... pigeon pea, etc. are currently the most important legumes for human consumption and animal feed. Amaranth and quinoa are considered “pseudocereals” and are also good sources of proteins. Amaranth seeds contain lysine, an essential amino acid, limited in other grains or plant sources but are limited ...
Polypeptide: alpha-helix and beta
... Concept: Peptide chains tend to form orderly hydrogen-bonded arrangements. Materials: alpha-helix and beta-sheet models made by Prof. Ewing Procedure: Models may be used to help explain secondary protein structure. Related Information: Fibrous proteins are stringy, tough, and usually insoluble in ...
... Concept: Peptide chains tend to form orderly hydrogen-bonded arrangements. Materials: alpha-helix and beta-sheet models made by Prof. Ewing Procedure: Models may be used to help explain secondary protein structure. Related Information: Fibrous proteins are stringy, tough, and usually insoluble in ...
Two Rules on Protein-Ligand Interactions Xiaodong Pang1, 2
... Understanding the ruling principles of interaction between a target protein and a ligand is of paramount importance in drug discovery efforts. So far, in finding a real ligand for a given target protein, we are limited to experimental screening from a large number of small molecules, or through free ...
... Understanding the ruling principles of interaction between a target protein and a ligand is of paramount importance in drug discovery efforts. So far, in finding a real ligand for a given target protein, we are limited to experimental screening from a large number of small molecules, or through free ...
Lecture 1
... When genes are expressed, the genetic information (base sequence) on DNA is first transcribed (copied) to a molecule of messenger RNA in a process similar to DNA replication. The mRNA molecules then leave the cell nucleus and enter the cytoplasm, where triplets of (codons) forming the genetic code ...
... When genes are expressed, the genetic information (base sequence) on DNA is first transcribed (copied) to a molecule of messenger RNA in a process similar to DNA replication. The mRNA molecules then leave the cell nucleus and enter the cytoplasm, where triplets of (codons) forming the genetic code ...
Anton Supercomputer, a computational microscope.
... pathways are traversed that are distinct in the sense that native interactions are formed in different orders and that the pathways do not interconvert on the transition path time scale. Examined the thermodynamics and kinetics of the folding process, and in particular the existence and size of the ...
... pathways are traversed that are distinct in the sense that native interactions are formed in different orders and that the pathways do not interconvert on the transition path time scale. Examined the thermodynamics and kinetics of the folding process, and in particular the existence and size of the ...
Document
... Denaturation/renaturation of domains ofa protein (titin) using the atomic force microscope. ...
... Denaturation/renaturation of domains ofa protein (titin) using the atomic force microscope. ...
Protein folding

Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil.Each protein exists as an unfolded polypeptide or random coil when translated from a sequence of mRNA to a linear chain of amino acids. This polypeptide lacks any stable (long-lasting) three-dimensional structure (the left hand side of the first figure). Amino acids interact with each other to produce a well-defined three-dimensional structure, the folded protein (the right hand side of the figure), known as the native state. The resulting three-dimensional structure is determined by the amino acid sequence (Anfinsen's dogma). Experiments beginning in the 1980s indicate the codon for an amino acid can also influence protein structure.The correct three-dimensional structure is essential to function, although some parts of functional proteins may remain unfolded, so that protein dynamics is important. Failure to fold into native structure generally produces inactive proteins, but in some instances misfolded proteins have modified or toxic functionality. Several neurodegenerative and other diseases are believed to result from the accumulation of amyloid fibrils formed by misfolded proteins. Many allergies are caused by incorrect folding of some proteins, because the immune system does not produce antibodies for certain protein structures.