Discussion Problem Set 3 C483 Spring 2014
... 1. What are the two main forces that stabilize an alpha helix? Describe them. How might you test the importance of an n-pi-star interaction? 2. What two things need to be minimized to have a stable alpha helix? 3. Which amino acid regularly adopts a cis peptide bond? Explain why this is possible. 4. ...
... 1. What are the two main forces that stabilize an alpha helix? Describe them. How might you test the importance of an n-pi-star interaction? 2. What two things need to be minimized to have a stable alpha helix? 3. Which amino acid regularly adopts a cis peptide bond? Explain why this is possible. 4. ...
Thermodynamics of Protein Folding
... – Large stabilization factors, large destabilization factors, but small difference between them – Use RNase T1 as a model for study (because structure is well known and many mutants have been studied) ...
... – Large stabilization factors, large destabilization factors, but small difference between them – Use RNase T1 as a model for study (because structure is well known and many mutants have been studied) ...
Globular proteins
... When a beam of X-ray of a given wave length falls on a crystal, the xrays are diffracted by the electrons of various atoms of the crystal. The diffracted X-rays are recorded on a photographic film or x-ray film by producing a pattern of spots with various intensities. By analysis of the x-ray diffra ...
... When a beam of X-ray of a given wave length falls on a crystal, the xrays are diffracted by the electrons of various atoms of the crystal. The diffracted X-rays are recorded on a photographic film or x-ray film by producing a pattern of spots with various intensities. By analysis of the x-ray diffra ...
Experience Canola Protein in Great-Tasting Products
... Experience Canola Protein in Great-Tasting Products A core foundation of Coalescence’s mission is creating products that are healthy, yet delicious. We ...
... Experience Canola Protein in Great-Tasting Products A core foundation of Coalescence’s mission is creating products that are healthy, yet delicious. We ...
Questions for Discussion or Assignment to Accompany the Ubiquitin
... comment upon its 1H and 15N chemical shifts. You should download the structure file from the protein data bank and examine it with Rasmol (pdbid : 1D3Z). Make a print out of the protein with ILE36 clearly indicated, such as with a different color. 6. Investigate the literature for ubiquitin to find ...
... comment upon its 1H and 15N chemical shifts. You should download the structure file from the protein data bank and examine it with Rasmol (pdbid : 1D3Z). Make a print out of the protein with ILE36 clearly indicated, such as with a different color. 6. Investigate the literature for ubiquitin to find ...
John Torri Basic Nutrition Special Topic: Protein November 13 2014
... As we have learned from our Nutrition class, we need a daily intake of carbohydrates, fats, and proteins. Most people don’t know how proteins are stored, sources of proteins, or even how they work. I found an article that helps shed light on this topic. According to “Choosing Protein Wisely” Our bod ...
... As we have learned from our Nutrition class, we need a daily intake of carbohydrates, fats, and proteins. Most people don’t know how proteins are stored, sources of proteins, or even how they work. I found an article that helps shed light on this topic. According to “Choosing Protein Wisely” Our bod ...
Document
... the sun through photosynthesis. Assuming that energy conversions are not 100 % efficient, the total biomass of animals should be less than that of plants. A group of individuals of the same species that interact is called a population; a number of such groups that live and interact in the same area ...
... the sun through photosynthesis. Assuming that energy conversions are not 100 % efficient, the total biomass of animals should be less than that of plants. A group of individuals of the same species that interact is called a population; a number of such groups that live and interact in the same area ...
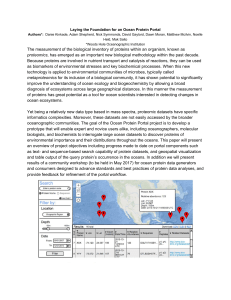
The measurement of the biological inventory of proteins within an
... *Woods Hole Oceanographic Institution ...
... *Woods Hole Oceanographic Institution ...
AS Biology - Everything Protein
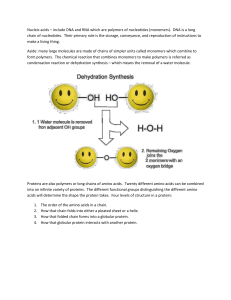
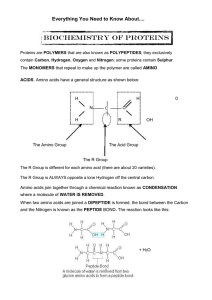
... where a molecule of WATER IS REMOVED. When two amino acids are joined a DIPEPTIDE is formed, the bond between the Carbon and the Nitrogen is known as the PEPTIDE BOND. The reaction looks like this: ...
... where a molecule of WATER IS REMOVED. When two amino acids are joined a DIPEPTIDE is formed, the bond between the Carbon and the Nitrogen is known as the PEPTIDE BOND. The reaction looks like this: ...
An Approach to Including Protein Quality When
... The production of protein from animal sources is often criticized because of the low efficiency of converting plant protein from feeds into protein in the animal products. However, this critique does not consider the fact that large portions of the plant-based proteins fed to animals may be human-in ...
... The production of protein from animal sources is often criticized because of the low efficiency of converting plant protein from feeds into protein in the animal products. However, this critique does not consider the fact that large portions of the plant-based proteins fed to animals may be human-in ...
ECS 189K - UC Davis
... http://www.rcsb.org, you can locate proteins by keyword searching or by entering the PDB accession number for the structure file, like 5PTI. Details on the molecule (how the structure was determined, pertinent research articles, position of secondary structures, unusual amino acids, etc) can be fou ...
... http://www.rcsb.org, you can locate proteins by keyword searching or by entering the PDB accession number for the structure file, like 5PTI. Details on the molecule (how the structure was determined, pertinent research articles, position of secondary structures, unusual amino acids, etc) can be fou ...
PROTEIN STRUCTURE ANALYSIS ASSIGNMENTS Search from
... What domains there are and what are their functions? Are there clefts of pores? What do they mean? Does the structure contain any metals? If so, which? In what reaction is the protein involved? Visualize your protein Test different visualizations and expaln for what purpose they could be used for Vi ...
... What domains there are and what are their functions? Are there clefts of pores? What do they mean? Does the structure contain any metals? If so, which? In what reaction is the protein involved? Visualize your protein Test different visualizations and expaln for what purpose they could be used for Vi ...
37151
... •Consist of 4 parallel metal rods •Two opposite rods have +ve potential and other two have – ve potential (AC & DC) •Applied voltage influence trajectory of ions traveling down flight path centered bw two rods •For given voltage, only certain m/z ions will reach detector ...
... •Consist of 4 parallel metal rods •Two opposite rods have +ve potential and other two have – ve potential (AC & DC) •Applied voltage influence trajectory of ions traveling down flight path centered bw two rods •For given voltage, only certain m/z ions will reach detector ...
Organelles and Cellular Function
... Relate cellular metabolism and transport to homeostasis and cellular reproduction. ► e. Describe how structure and function are related in terms of cell and tissue types. ...
... Relate cellular metabolism and transport to homeostasis and cellular reproduction. ► e. Describe how structure and function are related in terms of cell and tissue types. ...
ppt - Avraham Samson`s Lab
... number of degrees of freedom in a polypeptide chain, the molecule has an astronomical number of possible conformations. For example, a polypeptide of 100 residues will have 99 peptide bonds, and therefore 198 different phi and psi bond angles. If each of these bond angles can be in one of three stab ...
... number of degrees of freedom in a polypeptide chain, the molecule has an astronomical number of possible conformations. For example, a polypeptide of 100 residues will have 99 peptide bonds, and therefore 198 different phi and psi bond angles. If each of these bond angles can be in one of three stab ...
ab initio
... • “new view” of protein folding (Peter Wolynes) through funnels in the energy landscape • Not all sequences have unique native structure ...
... • “new view” of protein folding (Peter Wolynes) through funnels in the energy landscape • Not all sequences have unique native structure ...
Importance of Proteins PowerPoint
... Describe ways in which protein is used in food preparation. Identify the essential and nonessential amino acids. Compare and contrast complete and incomplete proteins. Explain what happens during the denaturation of protein and how the process occurs. Explain coagulation and apply basic principles o ...
... Describe ways in which protein is used in food preparation. Identify the essential and nonessential amino acids. Compare and contrast complete and incomplete proteins. Explain what happens during the denaturation of protein and how the process occurs. Explain coagulation and apply basic principles o ...
Clp proteins in photosynthetic organisms: An essential family of
... Clp proteins in photosynthetic organisms: An essential family of molecular chaperones and proteases Handledare: Adrian Clarke Molecular chaperones and proteases are vital for regulating the function and structure of most proteins within a cell. They are found in all organisms and are separated into ...
... Clp proteins in photosynthetic organisms: An essential family of molecular chaperones and proteases Handledare: Adrian Clarke Molecular chaperones and proteases are vital for regulating the function and structure of most proteins within a cell. They are found in all organisms and are separated into ...
ppt
... • ‘To understand the biological function of proteins we would .. Like to be able to deduce or predict the threedimensional structure from the amino acid sequence.’ • ‘This we cannot do.’ ...
... • ‘To understand the biological function of proteins we would .. Like to be able to deduce or predict the threedimensional structure from the amino acid sequence.’ • ‘This we cannot do.’ ...
The World of Chemistry Episode 24
... 3. Briefly describe the four types of protein structure. Primary - the sequence of amino acids in the protein chain Secondary - the formation of - helices or - sheets from the protein chain Tertiary - the folding of protein chains into more compact structures Quatrenary structure - the interacti ...
... 3. Briefly describe the four types of protein structure. Primary - the sequence of amino acids in the protein chain Secondary - the formation of - helices or - sheets from the protein chain Tertiary - the folding of protein chains into more compact structures Quatrenary structure - the interacti ...
Episode 24 - The Genetic Code
... 3. Briefly describe the four types of protein structure. Primary - the sequence of amino acids in the protein chain Secondary - the formation of - helices or - sheets from the protein chain Tertiary - the folding of protein chains into more compact structures Quatrenary structure - the interacti ...
... 3. Briefly describe the four types of protein structure. Primary - the sequence of amino acids in the protein chain Secondary - the formation of - helices or - sheets from the protein chain Tertiary - the folding of protein chains into more compact structures Quatrenary structure - the interacti ...
corriganpaperabstract - Workspace
... Nucleotide signalling molecules are important messengers in key pathways that allow cellular responses to changing environments. Canonical secondary signalling molecules act through specific receptor proteins by direct binding to alter their activity. Cyclic diadenosine monophosphate (c-di-AMP) is a ...
... Nucleotide signalling molecules are important messengers in key pathways that allow cellular responses to changing environments. Canonical secondary signalling molecules act through specific receptor proteins by direct binding to alter their activity. Cyclic diadenosine monophosphate (c-di-AMP) is a ...
Analytical Sciences, Poster AS-101 Kinetics and identification of non
... by pipets is the low reproducibility due to spreading and therefore the dilution of the sample. To overcome this, we developed a protocol to spray trypsin or matrix solution. This works in a short time and preserves the multiplexing SPRi measurements. As a proof of concept we will show an investigat ...
... by pipets is the low reproducibility due to spreading and therefore the dilution of the sample. To overcome this, we developed a protocol to spray trypsin or matrix solution. This works in a short time and preserves the multiplexing SPRi measurements. As a proof of concept we will show an investigat ...
Protein structure
... made up of such elements as carbon, hydrogen, nitrogen, phosphorus, oxygen, and sulfur. All proteins are polymers of amino acids. The polymers, also known as polypeptides consist of a sequence of 20 different L-α-amino acids, also referred to as residues. For chains under 40 residues the term peptid ...
... made up of such elements as carbon, hydrogen, nitrogen, phosphorus, oxygen, and sulfur. All proteins are polymers of amino acids. The polymers, also known as polypeptides consist of a sequence of 20 different L-α-amino acids, also referred to as residues. For chains under 40 residues the term peptid ...
Protein folding and structure
... 12. Ribonuclease structure is stabilized by ∆G = -7.1 kJ/mol at pH 2.5 and T = 25 ºC. What is the ratio between folded and denatured molecules? The enthalphy for folding is 238.6 kJ/mol at 25 ºC. Assume that enthalpy and entropy do not change when temperature is raised to 37 ºC. What is ∆G at 37 ºC ...
... 12. Ribonuclease structure is stabilized by ∆G = -7.1 kJ/mol at pH 2.5 and T = 25 ºC. What is the ratio between folded and denatured molecules? The enthalphy for folding is 238.6 kJ/mol at 25 ºC. Assume that enthalpy and entropy do not change when temperature is raised to 37 ºC. What is ∆G at 37 ºC ...
Protein folding

Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil.Each protein exists as an unfolded polypeptide or random coil when translated from a sequence of mRNA to a linear chain of amino acids. This polypeptide lacks any stable (long-lasting) three-dimensional structure (the left hand side of the first figure). Amino acids interact with each other to produce a well-defined three-dimensional structure, the folded protein (the right hand side of the figure), known as the native state. The resulting three-dimensional structure is determined by the amino acid sequence (Anfinsen's dogma). Experiments beginning in the 1980s indicate the codon for an amino acid can also influence protein structure.The correct three-dimensional structure is essential to function, although some parts of functional proteins may remain unfolded, so that protein dynamics is important. Failure to fold into native structure generally produces inactive proteins, but in some instances misfolded proteins have modified or toxic functionality. Several neurodegenerative and other diseases are believed to result from the accumulation of amyloid fibrils formed by misfolded proteins. Many allergies are caused by incorrect folding of some proteins, because the immune system does not produce antibodies for certain protein structures.