Document
... • C, H, O, N (sometimes Sulfer) • Monomer – amino acid • Amino acids link together via PEPTIDE bonds to form a polypeptide chain • This chain folds up to form a functional protein ...
... • C, H, O, N (sometimes Sulfer) • Monomer – amino acid • Amino acids link together via PEPTIDE bonds to form a polypeptide chain • This chain folds up to form a functional protein ...
Complex Protein Structure
... acids tend to orient inside the quaternary structure and away from the watery environment C) Hydrophilic amino acids tend to orient themselves outside the quaternary structure near the watery environment. ...
... acids tend to orient inside the quaternary structure and away from the watery environment C) Hydrophilic amino acids tend to orient themselves outside the quaternary structure near the watery environment. ...
Proteins are biopolymers construced from similar building blocks
... Semmelweis University, Dept. Biophysics and Radiation Biology Budapest Puskin u. 9. PF 263 H-1444 Hungary ...
... Semmelweis University, Dept. Biophysics and Radiation Biology Budapest Puskin u. 9. PF 263 H-1444 Hungary ...
20 Proteins - mrhortonbiology
... Mr. Horton, concerned about his wonderful students, has decided to help them out. I conducted a test for starch, sugar, and protein to try to determine what the food is (it isn’t necessarily one we tested in class). The starch test was brown but not black. The sugar and protein test are shown below. ...
... Mr. Horton, concerned about his wonderful students, has decided to help them out. I conducted a test for starch, sugar, and protein to try to determine what the food is (it isn’t necessarily one we tested in class). The starch test was brown but not black. The sugar and protein test are shown below. ...
Protein Domains
... Low complexity regions The individual domains of multidomain proteins are frequently separated from each other by regions of low complexity, also referred to as linker sequences Long stretches of repeated residues, particularly proline, glutamine, serine or threonine often indicate linker sequences ...
... Low complexity regions The individual domains of multidomain proteins are frequently separated from each other by regions of low complexity, also referred to as linker sequences Long stretches of repeated residues, particularly proline, glutamine, serine or threonine often indicate linker sequences ...
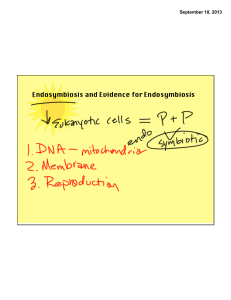
Endosymbiosis and Evidence for Endosymbiosis
... glands, airways, sinuses, ducts in the digestion and reproduction system. • Allows mucus to slide freely on these linings • Mutation= no hypertonic condition is established on the exterior of the cell and ...
... glands, airways, sinuses, ducts in the digestion and reproduction system. • Allows mucus to slide freely on these linings • Mutation= no hypertonic condition is established on the exterior of the cell and ...
Vegetarian- getting enough protein
... must ingest are called essential amino acids. A food that contains all nine essential amino acids is called a complete protein and is considered a high-value food. Animal proteins are complete proteins, as are a few plant foods, such as soy products and quinoa. If you are vegan and therefore choose ...
... must ingest are called essential amino acids. A food that contains all nine essential amino acids is called a complete protein and is considered a high-value food. Animal proteins are complete proteins, as are a few plant foods, such as soy products and quinoa. If you are vegan and therefore choose ...
The molecular architecture, macro-organization and functions of the
... diverse functions, which are also documented by ultrafast time-resolved spectroscopy studies. Hence, it is essential to have detailed and accurate information about the molecular architectures and macroorganizations of these protein complexes in vivo and in vitro in their different functional states ...
... diverse functions, which are also documented by ultrafast time-resolved spectroscopy studies. Hence, it is essential to have detailed and accurate information about the molecular architectures and macroorganizations of these protein complexes in vivo and in vitro in their different functional states ...
Cartoon modeling of proteins
... formation + motor protein separation + control sequences) Self assembly of viruses from their coat proteins ...
... formation + motor protein separation + control sequences) Self assembly of viruses from their coat proteins ...
Cartoon modeling of proteins
... formation + motor protein separation + control sequences) Self assembly of viruses from their coat proteins ...
... formation + motor protein separation + control sequences) Self assembly of viruses from their coat proteins ...
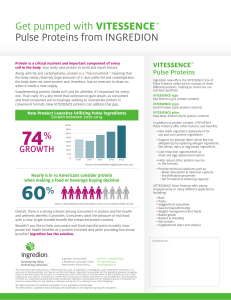
60% 74% - Ingredion
... Get pumped with VITESSENCE™ Pulse Proteins from INGREDION Protein is a critical nutrient and important component of every cell in the body. Your body uses protein to build and repair tissues. Along with fat and carbohydrates, protein is a “macronutrient,” meaning that the body needs relatively large ...
... Get pumped with VITESSENCE™ Pulse Proteins from INGREDION Protein is a critical nutrient and important component of every cell in the body. Your body uses protein to build and repair tissues. Along with fat and carbohydrates, protein is a “macronutrient,” meaning that the body needs relatively large ...
Most Proteins Don`t Exist!
... having a useful function, at least under the ambient conditions of the planet Earth. How lucky we are that some useful proteins have come into existence. This disturbing fact, that most proteins do not exist, was first brought to my attention by one of my lecturers, Dr. Alan Yarwood, when I was an u ...
... having a useful function, at least under the ambient conditions of the planet Earth. How lucky we are that some useful proteins have come into existence. This disturbing fact, that most proteins do not exist, was first brought to my attention by one of my lecturers, Dr. Alan Yarwood, when I was an u ...
Peptide Bonds
... Emil Fischer formulated the lock-and-key mechanism for enzymes All reactions which occur in living cells are mediated by enzymes and are catalysed by 106-108 Some enzymes may require the presence of a Cofactor. This may be a metal atom, which is essential for its redox activity. Others may require t ...
... Emil Fischer formulated the lock-and-key mechanism for enzymes All reactions which occur in living cells are mediated by enzymes and are catalysed by 106-108 Some enzymes may require the presence of a Cofactor. This may be a metal atom, which is essential for its redox activity. Others may require t ...
G Protein Coupled Receptors
... GTP exchange leads to a conformational change in the switch region. This leads to dissociation of Gß and it destabilizes the region where the G protein N- and Cterminus come together with the GPCR C-terminus, which leads to G protein dissociation. ...
... GTP exchange leads to a conformational change in the switch region. This leads to dissociation of Gß and it destabilizes the region where the G protein N- and Cterminus come together with the GPCR C-terminus, which leads to G protein dissociation. ...
6th semester-2006 Project Proposal
... disulfide bridge. This results in the generation of highly reactive charged cysteine residue which immediately cross-link to free sulfhydryl groups. The method is currently used to generate nano-arrays of technologically relevant proteins such as immunoglobulins, for medical diagnosis. The main draw ...
... disulfide bridge. This results in the generation of highly reactive charged cysteine residue which immediately cross-link to free sulfhydryl groups. The method is currently used to generate nano-arrays of technologically relevant proteins such as immunoglobulins, for medical diagnosis. The main draw ...
Theme 1 - NUI Galway
... binding affinity. The aim of this project is to investigate how small molecule modifications of the protein surface can influence that protein’s interaction behaviour. Proteins labelled with fluorescent dyes are used extensively in protein interaction studies both in vivo and in vitro. Frequently, s ...
... binding affinity. The aim of this project is to investigate how small molecule modifications of the protein surface can influence that protein’s interaction behaviour. Proteins labelled with fluorescent dyes are used extensively in protein interaction studies both in vivo and in vitro. Frequently, s ...
Naomi`s Nucleants - Molecular Dimensions
... surface with cavities of similar sizes to proteins. It is hypothesised that the cavities entrap protein molecules, thereby encouraging nucleation and crystal formation. ...
... surface with cavities of similar sizes to proteins. It is hypothesised that the cavities entrap protein molecules, thereby encouraging nucleation and crystal formation. ...
A1980JQ46200001
... inevitable that, in addition to my primary work on myosin, I became involved in studies already under way on tyrosyl peptides. In the late 1950s most biochemical labs had only limited physical chemical instrumentation, most likely pH meters and spectrophotometers. The attraction of ...
... inevitable that, in addition to my primary work on myosin, I became involved in studies already under way on tyrosyl peptides. In the late 1950s most biochemical labs had only limited physical chemical instrumentation, most likely pH meters and spectrophotometers. The attraction of ...
Title - Iowa State University
... B. increase the ΔG of a reaction C. decrease the Δ G of a reaction D. increase the activation energy of the reaction 9.) Polypeptide chains are always synthesized from _______ to _______. a.) hydrophobic to hydrophilic b.) N terminus to C terminus c.) The amino group to carboxyl group d.) Alpha heli ...
... B. increase the ΔG of a reaction C. decrease the Δ G of a reaction D. increase the activation energy of the reaction 9.) Polypeptide chains are always synthesized from _______ to _______. a.) hydrophobic to hydrophilic b.) N terminus to C terminus c.) The amino group to carboxyl group d.) Alpha heli ...
NAME
... 1. Check with the other groups in the class. What other variants of the gene exist? How similar or dissimilar were their DNA sequence? ...
... 1. Check with the other groups in the class. What other variants of the gene exist? How similar or dissimilar were their DNA sequence? ...
Rice Krispie Treats
... 1. Check with the other groups in the class. What other variants of the gene exist? How similar or dissimilar were their DNA sequence? ...
... 1. Check with the other groups in the class. What other variants of the gene exist? How similar or dissimilar were their DNA sequence? ...
Wrkshp04
... 44 pts 2) A protein will be least soluble in water when the pH = _____. The interaction of a protein side group which is acidic with one which is basic will form a ___________. Cysteine residues stabilize tertiary protein structure by forming: _______________________ . A group of hydrophobic side-gr ...
... 44 pts 2) A protein will be least soluble in water when the pH = _____. The interaction of a protein side group which is acidic with one which is basic will form a ___________. Cysteine residues stabilize tertiary protein structure by forming: _______________________ . A group of hydrophobic side-gr ...
Protein folding

Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil.Each protein exists as an unfolded polypeptide or random coil when translated from a sequence of mRNA to a linear chain of amino acids. This polypeptide lacks any stable (long-lasting) three-dimensional structure (the left hand side of the first figure). Amino acids interact with each other to produce a well-defined three-dimensional structure, the folded protein (the right hand side of the figure), known as the native state. The resulting three-dimensional structure is determined by the amino acid sequence (Anfinsen's dogma). Experiments beginning in the 1980s indicate the codon for an amino acid can also influence protein structure.The correct three-dimensional structure is essential to function, although some parts of functional proteins may remain unfolded, so that protein dynamics is important. Failure to fold into native structure generally produces inactive proteins, but in some instances misfolded proteins have modified or toxic functionality. Several neurodegenerative and other diseases are believed to result from the accumulation of amyloid fibrils formed by misfolded proteins. Many allergies are caused by incorrect folding of some proteins, because the immune system does not produce antibodies for certain protein structures.