Document
... other proteins and help them fold/assemble properly (can be folding of one protein and assembly of multiple proteins). Heat shock protein story: Two major types: type I includes hsp70---bind and prevent misfolding of the substrate proteins (can also unfold proteins)---cytosol, chloroplast, mitochond ...
... other proteins and help them fold/assemble properly (can be folding of one protein and assembly of multiple proteins). Heat shock protein story: Two major types: type I includes hsp70---bind and prevent misfolding of the substrate proteins (can also unfold proteins)---cytosol, chloroplast, mitochond ...
Protein Targeting
... Peptide bond formation is thermodynamically unfavourable and therefore amino acids are charged ...
... Peptide bond formation is thermodynamically unfavourable and therefore amino acids are charged ...
2.22 Protein Synthesis.docx
... polypeptide. As shown below, this is a fairly involved process. DNA contains the genetic code that is used as a template to create mRNA in a process known as transcription. The mRNA then moves out of the nucleus into the cytoplasm where it serves as the template for translation, where tRNAs bring in ...
... polypeptide. As shown below, this is a fairly involved process. DNA contains the genetic code that is used as a template to create mRNA in a process known as transcription. The mRNA then moves out of the nucleus into the cytoplasm where it serves as the template for translation, where tRNAs bring in ...
The Essential Need for Protein Chemists
... is associated with loss of function and (under normal physiological conditions) is higher in Gibbs energy. The transition between these states is a physical process (modulated by heat, pH or solutes), and thus potentially reversible, with the partition function defined by the Gibbs energy difference ...
... is associated with loss of function and (under normal physiological conditions) is higher in Gibbs energy. The transition between these states is a physical process (modulated by heat, pH or solutes), and thus potentially reversible, with the partition function defined by the Gibbs energy difference ...
Prob_Set_2_2007
... four-helix bundle. If you choose a bundle, things to consider are: length of the helical segments (check recent work from the M. H. Hecht lab), residues that favor helical structure, construction of amphipathic helices, appropriate sequences for turns, selection of residues exposed to the aqueous me ...
... four-helix bundle. If you choose a bundle, things to consider are: length of the helical segments (check recent work from the M. H. Hecht lab), residues that favor helical structure, construction of amphipathic helices, appropriate sequences for turns, selection of residues exposed to the aqueous me ...
Protein Synthesis
... • A gene is piece of DNA that codes to make a protein. • Proteins play specific roles in an organism. – Support (elastin) – Transport (hemoglobin) – Control (hormones, insulin) – Immunity (antibodies) – Catalysis (enzymes) ...
... • A gene is piece of DNA that codes to make a protein. • Proteins play specific roles in an organism. – Support (elastin) – Transport (hemoglobin) – Control (hormones, insulin) – Immunity (antibodies) – Catalysis (enzymes) ...
SOLUGEL Protein Gummies Leaflet
... collagen protein in each gummy Triple your gummies’ protein content with SOLUGEL®! The traditional gummy bear contains around 6g of protein per 100g, entirely from its gelatin content. With SOLUGEL®, it is now possible to create a gummy rich in collagen protein that looks and tastes like any other g ...
... collagen protein in each gummy Triple your gummies’ protein content with SOLUGEL®! The traditional gummy bear contains around 6g of protein per 100g, entirely from its gelatin content. With SOLUGEL®, it is now possible to create a gummy rich in collagen protein that looks and tastes like any other g ...
Grand challenges in bioinformatics.
... from its amino acid sequence. It is widely believed that the amino acid sequence contains all the necessary information to make up the correct three-dimensional structure, since the protein folding is apparently thermodynamically determined; namely, given a proper environment, a protein would fold u ...
... from its amino acid sequence. It is widely believed that the amino acid sequence contains all the necessary information to make up the correct three-dimensional structure, since the protein folding is apparently thermodynamically determined; namely, given a proper environment, a protein would fold u ...
Structural Genomics
... The initial stages of folding must be nearly random, but if the entire process was a random search it would require too much time. Consider a 100 residue protein. If each residue is considered to have just 3 possible conformations the total number of conformations of the protein is 3100. Conformatio ...
... The initial stages of folding must be nearly random, but if the entire process was a random search it would require too much time. Consider a 100 residue protein. If each residue is considered to have just 3 possible conformations the total number of conformations of the protein is 3100. Conformatio ...
Proteomics techniques used to identify proteins
... – Cutting out the gel spot and trypsinizing the protein – Run LS/MS/MS on LTQ XL – Identify proteins by matching the tryptic peptides to theoretical fragments (Protein Database search) ...
... – Cutting out the gel spot and trypsinizing the protein – Run LS/MS/MS on LTQ XL – Identify proteins by matching the tryptic peptides to theoretical fragments (Protein Database search) ...
Proteins
... 6. Label the type of bond used to make proteins. 7. Draw arrows to identify these bonds in your model 8. Label the N-terminus and C-terminus 9. Put SQUARES around the R groups 10. Use your amino acid chart to identify & label the type of R group (non-polar, polar, charge basic, charged acidic, etc) ...
... 6. Label the type of bond used to make proteins. 7. Draw arrows to identify these bonds in your model 8. Label the N-terminus and C-terminus 9. Put SQUARES around the R groups 10. Use your amino acid chart to identify & label the type of R group (non-polar, polar, charge basic, charged acidic, etc) ...
AASK Additional Activities
... (native structure) following their complete unfolding (denaturation) by heating. 1. Have each student document the native shape of their folded protein with a digital photo. 2. Ask the students to unfold their protein and then re-fold it. 3. Check the refolded protein against the photo of the native ...
... (native structure) following their complete unfolding (denaturation) by heating. 1. Have each student document the native shape of their folded protein with a digital photo. 2. Ask the students to unfold their protein and then re-fold it. 3. Check the refolded protein against the photo of the native ...
here - BioGeometry
... which the whole system does nothing but vibration most of the time, and then occasionally something large moves,” he said. Also, Edelsbrunner said, there are few shortcuts to developing the complex simulation techniques. “The most difficulty we have with our software is that it is so labor-intensive ...
... which the whole system does nothing but vibration most of the time, and then occasionally something large moves,” he said. Also, Edelsbrunner said, there are few shortcuts to developing the complex simulation techniques. “The most difficulty we have with our software is that it is so labor-intensive ...
elastin - MBBS Students Club
... PrPSc has the same amino acid sequence as the normal protein; that is, their primary structures are identical but its secondary structure is dominated by beta conformation is insoluble in all but the strongest solvents is highly resistant to digestion by proteases ...
... PrPSc has the same amino acid sequence as the normal protein; that is, their primary structures are identical but its secondary structure is dominated by beta conformation is insoluble in all but the strongest solvents is highly resistant to digestion by proteases ...
ELASTIN - Rihs.com.pk
... PrPSc has the same amino acid sequence as the normal protein; that is, their primary structures are identical but its secondary structure is dominated by beta conformation is insoluble in all but the strongest solvents is highly resistant to digestion by proteases ...
... PrPSc has the same amino acid sequence as the normal protein; that is, their primary structures are identical but its secondary structure is dominated by beta conformation is insoluble in all but the strongest solvents is highly resistant to digestion by proteases ...
bioposter
... Cluster Based Folding and Future Aspects of Folding Use cluster computing as the testing ground for truly parallel simulations Individual proteins are discrete units Allows the program to be refined while highly parallel desktop computing comes to fruition ...
... Cluster Based Folding and Future Aspects of Folding Use cluster computing as the testing ground for truly parallel simulations Individual proteins are discrete units Allows the program to be refined while highly parallel desktop computing comes to fruition ...
http://gslc. genetics. utah.edu/units/basics/transcribe/
... http:// gslc. genetics. utah.edu/units/basics/transcribe/ Defme the following terms: Transcription, Translation, Codon Complete the "Build a Protein" Activity You will need to record the sequence of bases in the mRNA as well as the sequence of amino acids on a separate piece of paper that I will col ...
... http:// gslc. genetics. utah.edu/units/basics/transcribe/ Defme the following terms: Transcription, Translation, Codon Complete the "Build a Protein" Activity You will need to record the sequence of bases in the mRNA as well as the sequence of amino acids on a separate piece of paper that I will col ...
Protein Synthesis Drawing
... More tRNA molecules transfer correct amino acids to the growing protein chain (by matching the anticodon on tRNA to the codons on mRNA). Remember: One tRNA only carries one kind of A.A. ...
... More tRNA molecules transfer correct amino acids to the growing protein chain (by matching the anticodon on tRNA to the codons on mRNA). Remember: One tRNA only carries one kind of A.A. ...
Structural Genomics - University of Houston
... The initial stages of folding must be nearly random, but if the entire process was a random search it would require too much time. Consider a 100 residue protein. If each residue is considered to have just 3 possible conformations the total number of conformations of the protein is 3100. Conformatio ...
... The initial stages of folding must be nearly random, but if the entire process was a random search it would require too much time. Consider a 100 residue protein. If each residue is considered to have just 3 possible conformations the total number of conformations of the protein is 3100. Conformatio ...
Presentazione standard di PowerPoint
... Molecular dynamics simulations are a powerful tool to investigate the structural features of proteins at atomic level, and in particular to introduce flexibility and temporal evolution in the analysis of molecular systems. During these years I have been involved in the study of many different protei ...
... Molecular dynamics simulations are a powerful tool to investigate the structural features of proteins at atomic level, and in particular to introduce flexibility and temporal evolution in the analysis of molecular systems. During these years I have been involved in the study of many different protei ...
Protein Structure
... Protein Overall Structure Primary (1ᵒ): Amino Acids in a chain/sequence Secondary (2ᵒ): Alpha helix or Beta pleated chain Tertiary (3ᵒ): Complex shape Quaternary (4ᵒ): 2 or more proteins folded on each other ...
... Protein Overall Structure Primary (1ᵒ): Amino Acids in a chain/sequence Secondary (2ᵒ): Alpha helix or Beta pleated chain Tertiary (3ᵒ): Complex shape Quaternary (4ᵒ): 2 or more proteins folded on each other ...
Protein Needs for Athletes
... • Animal-derived proteins (milk, eggs, meat and fish) are high quality because they have all of the essential amino acids (EAAs), which are building blocks for proteins in our body. • Some plant-based proteins (soy, quinoa, amaranth, and buckwheat) contain all EAAs while most plant-bas ...
... • Animal-derived proteins (milk, eggs, meat and fish) are high quality because they have all of the essential amino acids (EAAs), which are building blocks for proteins in our body. • Some plant-based proteins (soy, quinoa, amaranth, and buckwheat) contain all EAAs while most plant-bas ...
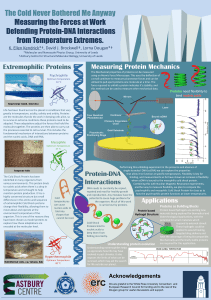
The Cold Never Bothered Me Anyway Measuring the Forces at Work
... temperature and is thought to help maintain protein production. It has a highly conserved structure but small differences in the amino acid sequence of extremophilic Cold Shock proteins change their flexibility, allowing them to move about and operate at the environment temperature of the organism. ...
... temperature and is thought to help maintain protein production. It has a highly conserved structure but small differences in the amino acid sequence of extremophilic Cold Shock proteins change their flexibility, allowing them to move about and operate at the environment temperature of the organism. ...
Protein folding

Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil.Each protein exists as an unfolded polypeptide or random coil when translated from a sequence of mRNA to a linear chain of amino acids. This polypeptide lacks any stable (long-lasting) three-dimensional structure (the left hand side of the first figure). Amino acids interact with each other to produce a well-defined three-dimensional structure, the folded protein (the right hand side of the figure), known as the native state. The resulting three-dimensional structure is determined by the amino acid sequence (Anfinsen's dogma). Experiments beginning in the 1980s indicate the codon for an amino acid can also influence protein structure.The correct three-dimensional structure is essential to function, although some parts of functional proteins may remain unfolded, so that protein dynamics is important. Failure to fold into native structure generally produces inactive proteins, but in some instances misfolded proteins have modified or toxic functionality. Several neurodegenerative and other diseases are believed to result from the accumulation of amyloid fibrils formed by misfolded proteins. Many allergies are caused by incorrect folding of some proteins, because the immune system does not produce antibodies for certain protein structures.