Elise Young: Animal & Range Sciences
... Linking common factors in the phenomenon of protein clumping observed in several diseases Proteins perform many important functions at the cellular level. However, if proteins do not fold properly, they are prone to aggregating and sticking together, preventing them from performing their functions, ...
... Linking common factors in the phenomenon of protein clumping observed in several diseases Proteins perform many important functions at the cellular level. However, if proteins do not fold properly, they are prone to aggregating and sticking together, preventing them from performing their functions, ...
College 5
... (meaning contacts that also occur in the functional protein). The potential energy drives the system to a conformation where a certain number of native contacts has been established, but the chain is not yet folded. Note that there are many possible pathways. Once this point is reached, the chain fo ...
... (meaning contacts that also occur in the functional protein). The potential energy drives the system to a conformation where a certain number of native contacts has been established, but the chain is not yet folded. Note that there are many possible pathways. Once this point is reached, the chain fo ...
A One- or Two-Day Course for Your Campus on
... ligands, substrates, and drugs, and protein evolutionary conservation. Handson experience will be largely with molecules of each participant's choosing. Participants will learn easy methods for creating publication-quality molecular images, and how to put snapshots or rotating animations in Powerpoi ...
... ligands, substrates, and drugs, and protein evolutionary conservation. Handson experience will be largely with molecules of each participant's choosing. Participants will learn easy methods for creating publication-quality molecular images, and how to put snapshots or rotating animations in Powerpoi ...
Protein Folding Questions only
... functional group. - Hydrophilic sidechains have various combinations of ____________. An exception to this observation is: ...
... functional group. - Hydrophilic sidechains have various combinations of ____________. An exception to this observation is: ...
D6- Bulletin Board Powerful Protein
... • There are 9 essential amino acids that our bodies can’t make, so we need to get them from our food. • If a protein food has all 9 essential amino acids, it is called a complete protein. If it doesn’t, it is called an incomplete protein. • You can eat incomplete protein foods together to make sure ...
... • There are 9 essential amino acids that our bodies can’t make, so we need to get them from our food. • If a protein food has all 9 essential amino acids, it is called a complete protein. If it doesn’t, it is called an incomplete protein. • You can eat incomplete protein foods together to make sure ...
Combinatorial docking approach for structure prediction of large
... calculations to build determine native conformations of larger protein complexes. Such an algorithm is much faster than trying to determine the entire protein or assembly structure through molecular dynamics methods that compute quantum physics interactions between each atom. Such structural informa ...
... calculations to build determine native conformations of larger protein complexes. Such an algorithm is much faster than trying to determine the entire protein or assembly structure through molecular dynamics methods that compute quantum physics interactions between each atom. Such structural informa ...
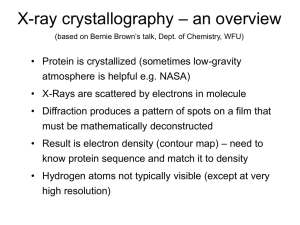
1. Overview
... – Heavy atom replacement – make a landmark – Ex: Selenomethionine • Plenty of computer algorithms now ...
... – Heavy atom replacement – make a landmark – Ex: Selenomethionine • Plenty of computer algorithms now ...
Proteins - Boardworks
... There are up to four levels of structure in a protein: primary, secondary, tertiary and quaternary. Each of these play an important role in the overall structure and function of the protein. 6 of 8 ...
... There are up to four levels of structure in a protein: primary, secondary, tertiary and quaternary. Each of these play an important role in the overall structure and function of the protein. 6 of 8 ...
Biosynthesis and degradation of proteins
... Protease inhibitors • IAPs are proteins that block apoptosis by binding to and inhibiting caspases. The apoptosis-stimulating protein Smac antagonizes the effect of IAPs on caspases. • TIMPs are inhibitors of metalloproteases that are secreted by cells. A domain of the inhibitor protein interacts w ...
... Protease inhibitors • IAPs are proteins that block apoptosis by binding to and inhibiting caspases. The apoptosis-stimulating protein Smac antagonizes the effect of IAPs on caspases. • TIMPs are inhibitors of metalloproteases that are secreted by cells. A domain of the inhibitor protein interacts w ...
Amino acid sequence fingerprints in divergent evolution of
... structure of a protein is more conserved than its primary structure. The tertiary structure is able to accommodate various sequence amendments without a substantial change of the spatial arrangement of the protein molecule so that if the essential residues of the active site are preserved, the resul ...
... structure of a protein is more conserved than its primary structure. The tertiary structure is able to accommodate various sequence amendments without a substantial change of the spatial arrangement of the protein molecule so that if the essential residues of the active site are preserved, the resul ...
Biochemistry- Ch 11. Carbohydrates
... cell-surface glycoproteins. The viral protein that binds to these sugars is called hemagglutinin. ...
... cell-surface glycoproteins. The viral protein that binds to these sugars is called hemagglutinin. ...
Slide 1
... aggregation, to facilitate their folding and to promote association with other subunits •Ribosomal proteins seem to play a role in recruiting chaperones e.g. in E coli the trigger protein associates with a ribosomal protein located at the outlet of the peptide exit tunnel •Trigger recognizes relativ ...
... aggregation, to facilitate their folding and to promote association with other subunits •Ribosomal proteins seem to play a role in recruiting chaperones e.g. in E coli the trigger protein associates with a ribosomal protein located at the outlet of the peptide exit tunnel •Trigger recognizes relativ ...
Spectroscopy of Proteins
... genes, translated form genes (mutation in gene leads to a mutated protein) • Made of a verity of 20 amino acid building blocks • Exert all the biological functions of the organism: enzymes, antibodies, cytoskeletons, hormones, receptors ...
... genes, translated form genes (mutation in gene leads to a mutated protein) • Made of a verity of 20 amino acid building blocks • Exert all the biological functions of the organism: enzymes, antibodies, cytoskeletons, hormones, receptors ...
Protein Folding and Quality Control
... Protein Folding and Quality Control Folding Function: making specific functional domains critical for function (occurs following or coincident with synthesis) Sequence dependence: Final structure of protein is dependent on amino acid sequence and properties of amino acids that make up polypeptide be ...
... Protein Folding and Quality Control Folding Function: making specific functional domains critical for function (occurs following or coincident with synthesis) Sequence dependence: Final structure of protein is dependent on amino acid sequence and properties of amino acids that make up polypeptide be ...
6. 3-D structure of proteins
... called its conformation. • Proteins in any of their functional folded conformations are called native proteins. • Stability can be defined as the tendency to maintain a native confirmation. • When water surrounds a hydrophobic molecule, the optimal arrangement of hydrogen bonds results in a highly s ...
... called its conformation. • Proteins in any of their functional folded conformations are called native proteins. • Stability can be defined as the tendency to maintain a native confirmation. • When water surrounds a hydrophobic molecule, the optimal arrangement of hydrogen bonds results in a highly s ...
Protein Engineering
... Aspargine Changes If asparagine and glutamine present in protein when heated, ammonia is released amino acids convert to aspartic acid and glutamic acid Protein may refold LOSE ACTIVITY ...
... Aspargine Changes If asparagine and glutamine present in protein when heated, ammonia is released amino acids convert to aspartic acid and glutamic acid Protein may refold LOSE ACTIVITY ...
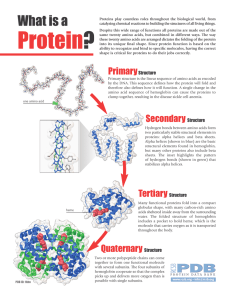
Protein?
... Alpha helices (shown in blue) are the basic structural elements found in hemoglobin, but many other proteins also include beta sheets. The inset highlights the pattern of hydrogen bonds (shown in green) that stabilizes alpha helices. ...
... Alpha helices (shown in blue) are the basic structural elements found in hemoglobin, but many other proteins also include beta sheets. The inset highlights the pattern of hydrogen bonds (shown in green) that stabilizes alpha helices. ...
Biological Macromolecules Worksheet
... a. the number _____ of different nitrogenous bases in DNA b. the number _____ of different chemical classes of amino acids c. the number _____ of chains of nucleotides in a DNA molecule d. the number _____ of different nitrogenous bases in RNA e. the number _____ of different amino acids found in pr ...
... a. the number _____ of different nitrogenous bases in DNA b. the number _____ of different chemical classes of amino acids c. the number _____ of chains of nucleotides in a DNA molecule d. the number _____ of different nitrogenous bases in RNA e. the number _____ of different amino acids found in pr ...
what are proteins? - scie
... therefore the function, of a protein depends entirely on the amino acid sequence. During digestion, proteins undergo hydrolysis and are split up into their component amino acids. The body can then use these as building blocks to make the proteins it needs. ...
... therefore the function, of a protein depends entirely on the amino acid sequence. During digestion, proteins undergo hydrolysis and are split up into their component amino acids. The body can then use these as building blocks to make the proteins it needs. ...
File
... • Certain proteins possess a fourth level of structural organization, quaternary structure. • Quaternary structures are found in proteins that consist of more than one polypeptide chain linked together. • Alternatively, proteins may have a quaternary structure of they include organic prosthetic grou ...
... • Certain proteins possess a fourth level of structural organization, quaternary structure. • Quaternary structures are found in proteins that consist of more than one polypeptide chain linked together. • Alternatively, proteins may have a quaternary structure of they include organic prosthetic grou ...
proteins——Echo,Jason,Philip
... B)make up cell membrane C)make up genetic material D)the main energy for organism ...
... B)make up cell membrane C)make up genetic material D)the main energy for organism ...
Chapter 5 – Proteins and Amino Acids
... 3. High-Quality Proteins 4. Complementary Proteins B. Protein Sparing Nutrition in Practice – Vegetarian Diets A. Are vegetarian diets nutritionally sound? B. What should be my main concerns when planning a nutritionally sound vegetarian diet? C. Isn’t protein a problem in vegetarian diets? D. What ...
... 3. High-Quality Proteins 4. Complementary Proteins B. Protein Sparing Nutrition in Practice – Vegetarian Diets A. Are vegetarian diets nutritionally sound? B. What should be my main concerns when planning a nutritionally sound vegetarian diet? C. Isn’t protein a problem in vegetarian diets? D. What ...
Growth Hormone Releasing Hormone (GHRH) - Cloud
... Accurate Molecular Mass: 43.5kDa as determined by SDS-PAGE reducing conditions. Applications: SDS-PAGE; WB; ELISA; IP. (May be suitable for use in other assays to be determined by the end user.) Note: The possible reasons that the actual band size differs from the predicted are as follows: 1. Splice ...
... Accurate Molecular Mass: 43.5kDa as determined by SDS-PAGE reducing conditions. Applications: SDS-PAGE; WB; ELISA; IP. (May be suitable for use in other assays to be determined by the end user.) Note: The possible reasons that the actual band size differs from the predicted are as follows: 1. Splice ...
View attached file
... 'Conformational diseases' are diseases caused by misfolding of a protein, often as a result of a missense mutation that does not necessarily disrupt the active site of the protein. As a result, the protein may lose its function, and often the misfolded monomers self-assemble to form cytotoxic aggreg ...
... 'Conformational diseases' are diseases caused by misfolding of a protein, often as a result of a missense mutation that does not necessarily disrupt the active site of the protein. As a result, the protein may lose its function, and often the misfolded monomers self-assemble to form cytotoxic aggreg ...
Protein folding

Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil.Each protein exists as an unfolded polypeptide or random coil when translated from a sequence of mRNA to a linear chain of amino acids. This polypeptide lacks any stable (long-lasting) three-dimensional structure (the left hand side of the first figure). Amino acids interact with each other to produce a well-defined three-dimensional structure, the folded protein (the right hand side of the figure), known as the native state. The resulting three-dimensional structure is determined by the amino acid sequence (Anfinsen's dogma). Experiments beginning in the 1980s indicate the codon for an amino acid can also influence protein structure.The correct three-dimensional structure is essential to function, although some parts of functional proteins may remain unfolded, so that protein dynamics is important. Failure to fold into native structure generally produces inactive proteins, but in some instances misfolded proteins have modified or toxic functionality. Several neurodegenerative and other diseases are believed to result from the accumulation of amyloid fibrils formed by misfolded proteins. Many allergies are caused by incorrect folding of some proteins, because the immune system does not produce antibodies for certain protein structures.