DIAGNOSTIC RELEVANCE OF PREDICTED ANTIGENIC
... bioinformatics analysis. Recombinant genes encoded selected amino acids sequences have been constructed from synthetic oligonucleotides by using PCR reaction. Proteins were expressed in E.coli as hybrid protein with Glutathione Stransferase and tested individually by enzyme immunoassay against a pan ...
... bioinformatics analysis. Recombinant genes encoded selected amino acids sequences have been constructed from synthetic oligonucleotides by using PCR reaction. Proteins were expressed in E.coli as hybrid protein with Glutathione Stransferase and tested individually by enzyme immunoassay against a pan ...
投影片 1
... Newly synthesized proteins in the living cell must go through a folding process to attain their functional structure. To achieve this in an efficient fashion, all organisms, including humans, have evolved a large set of molecular chaperones that assist the folding as well as the maintenance of the ...
... Newly synthesized proteins in the living cell must go through a folding process to attain their functional structure. To achieve this in an efficient fashion, all organisms, including humans, have evolved a large set of molecular chaperones that assist the folding as well as the maintenance of the ...
Proteins - Downtown Magnets High School
... Amino Acid Polymers • Amino acids are linked by peptide bonds ...
... Amino Acid Polymers • Amino acids are linked by peptide bonds ...
Proteins and amino acids
... Hydrophobicity – Protein structure Secondary structure elements tend to have one side turned to the surface (hydrophilic) one side turned to the core (hydrophobic) ...
... Hydrophobicity – Protein structure Secondary structure elements tend to have one side turned to the surface (hydrophilic) one side turned to the core (hydrophobic) ...
Macromolecules
... Secondary structure – helices and pleated sheet structures seen in proteins Interactions: H-bonds between H of one amino acid and O of nonadjacent a.a.s ...
... Secondary structure – helices and pleated sheet structures seen in proteins Interactions: H-bonds between H of one amino acid and O of nonadjacent a.a.s ...
In general, animal proteins are considered complete proteins. A complete... essential amino acids. Vegetable (plant-based) proteins are considered incomplete proteins...
... what protein sources you eat. A vegetarian can acquire the recommended amount of protein with a method known as complimentary protein, where you combine certain foods that will create a complete protein. For more information email: [email protected] ...
... what protein sources you eat. A vegetarian can acquire the recommended amount of protein with a method known as complimentary protein, where you combine certain foods that will create a complete protein. For more information email: [email protected] ...
DLS-Characterisation of protein melting point
... melting point Proteins are composed of polypeptide chains, synthesized within the cell from a pool of 20 different amino acid types. In contrast to manmade and random coil biological polymers, the protein’s polypeptide chains are folded into unique 3-dimensional structures in the natured state. Thes ...
... melting point Proteins are composed of polypeptide chains, synthesized within the cell from a pool of 20 different amino acid types. In contrast to manmade and random coil biological polymers, the protein’s polypeptide chains are folded into unique 3-dimensional structures in the natured state. Thes ...
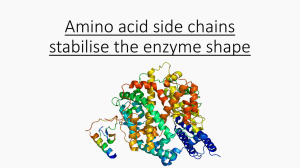
Amino acid side chains stabilise the enzyme shape Hydrogen bonds
... Many amino acids contain groups in the side chains that have a hydrogen atom attached to either an oxygen or nitrogen atom. Hydrogen bonding can occur between such groups. ...
... Many amino acids contain groups in the side chains that have a hydrogen atom attached to either an oxygen or nitrogen atom. Hydrogen bonding can occur between such groups. ...
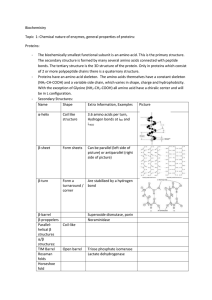
Biochemistry Topic 1: Chemical nature of enzymes, general
... structure (ΔGF < ΔGD). The 3D structure depends on the primary structure and if there has ben an error during translation or transcribtion or there has been a DNA mutatuin the most stable 3d structure might not be achieved this might result in a less or even inactive protein. Hydrophobic amino acids ...
... structure (ΔGF < ΔGD). The 3D structure depends on the primary structure and if there has ben an error during translation or transcribtion or there has been a DNA mutatuin the most stable 3d structure might not be achieved this might result in a less or even inactive protein. Hydrophobic amino acids ...
Chapter 20 Amino acids and proteins
... 2. Briefly, summarize the process of electrophoresis. 3. Given three or four amino acids, their pIs, and the pH of the buffer, determine the movement of the amino acids on an electrophoresis gel. 20.4 formation of peptides 1. Draw the structure of a dipetide from the zwitterions of two or more amino ...
... 2. Briefly, summarize the process of electrophoresis. 3. Given three or four amino acids, their pIs, and the pH of the buffer, determine the movement of the amino acids on an electrophoresis gel. 20.4 formation of peptides 1. Draw the structure of a dipetide from the zwitterions of two or more amino ...
Proteins Behaving badly - The University of Oklahoma
... fibrillar morphology and a regular cross-b sheet structure is linked to a number of neurodegenerative diseases. These aggregates, called amyloid, are believed to be the root cause of disease pathology. Although it was once believed that the insoluble aggregates were the biologically active species, ...
... fibrillar morphology and a regular cross-b sheet structure is linked to a number of neurodegenerative diseases. These aggregates, called amyloid, are believed to be the root cause of disease pathology. Although it was once believed that the insoluble aggregates were the biologically active species, ...
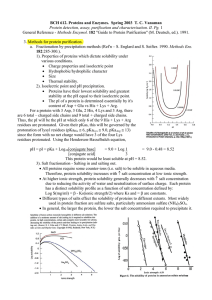
General Reference - Methods Enzymol. 182 "Guide to Protein
... x Charge properties and isoelectric point x Hydrophobic/hydrophilic character x Size x Thermal stability. 2). Isoelectric point and pH precipitation. x Proteins have their lowest solubility and greatest stability at the pH equal to their isoelectric point. x The pI of a protein is determined essenti ...
... x Charge properties and isoelectric point x Hydrophobic/hydrophilic character x Size x Thermal stability. 2). Isoelectric point and pH precipitation. x Proteins have their lowest solubility and greatest stability at the pH equal to their isoelectric point. x The pI of a protein is determined essenti ...
Presentazione di PowerPoint
... • occurrence of lignocellulosic fibers avoids the complete coagulation of proteins and facilitates processes like extrusion or injection-molding • mechanical properties of oil cake-based materials are lower than for similar starch-based composites but they possess a natural resistance to moisture th ...
... • occurrence of lignocellulosic fibers avoids the complete coagulation of proteins and facilitates processes like extrusion or injection-molding • mechanical properties of oil cake-based materials are lower than for similar starch-based composites but they possess a natural resistance to moisture th ...
Crystallizing a clearer understanding of the protein
... Jörg Stetefeld’s discoveries on the structure and function of proteins are a starting point for developing new drugs and other biotechnologies They’ve been described as the workhorses of life at the cellular level. Given the array of intelligent functions that proteins conduct in organisms, however, ...
... Jörg Stetefeld’s discoveries on the structure and function of proteins are a starting point for developing new drugs and other biotechnologies They’ve been described as the workhorses of life at the cellular level. Given the array of intelligent functions that proteins conduct in organisms, however, ...
Survey of Protein Structure Prediction Methods
... interactions and close packing; hydrogen bonding has little effect Beta-sheets stabilized by non-polar interactions between residues on adjacent strands Work supports idea that SSEs coded for locally in the sequence ...
... interactions and close packing; hydrogen bonding has little effect Beta-sheets stabilized by non-polar interactions between residues on adjacent strands Work supports idea that SSEs coded for locally in the sequence ...
Effect of protein aggregation and protein structure on magnetite
... method normally used to prevent the aggregation of integral membrane proteins is the introduction of detergents during protein purification. In this study, results from protein aggregation following the addition of three detergents are presented. Magnetite particles formed in the presence of MamC pu ...
... method normally used to prevent the aggregation of integral membrane proteins is the introduction of detergents during protein purification. In this study, results from protein aggregation following the addition of three detergents are presented. Magnetite particles formed in the presence of MamC pu ...
Chapter 1 I am - Mrs Smith`s Biology
... I am the class of protein formed by several spiral-shaped polypeptide molecules becoming linked together in parallel by cross-bridges, giving the protein a rope-like structure Amino Acids ...
... I am the class of protein formed by several spiral-shaped polypeptide molecules becoming linked together in parallel by cross-bridges, giving the protein a rope-like structure Amino Acids ...
Chapter 5: Biological Molecules Molecules of Life • All life made up
... o Inherited blood disorder o Single amino acid change in protein hemoglobin Amino Acid 6 is Valine instead of Glutamic Acid Alters shape & function Protein Structure o Physical & chemical conditions affect structure, along w/ primary structure Changes in pH, salt, temp, or other environmenta ...
... o Inherited blood disorder o Single amino acid change in protein hemoglobin Amino Acid 6 is Valine instead of Glutamic Acid Alters shape & function Protein Structure o Physical & chemical conditions affect structure, along w/ primary structure Changes in pH, salt, temp, or other environmenta ...
Protein functions part 2 File
... The alpha helix forms when the linear chain coils into a right handed helix The beta pleated sheet forms when the linear chain folds back on itself many times Hydrogen bonds play a major part in stabilising the secondary structure of proteins Many proteins bend and fold further to form globu ...
... The alpha helix forms when the linear chain coils into a right handed helix The beta pleated sheet forms when the linear chain folds back on itself many times Hydrogen bonds play a major part in stabilising the secondary structure of proteins Many proteins bend and fold further to form globu ...
MCB100A/CHEM130A In-Section Quiz #2 (Aathavan Karunakaran)
... Catalytic site (functionally important residues – often very very specific) Hydrophobic core (tolerates most hydrobic residues, and polar uncharged residues) Hydrophilic Surface (tolerates almost all sorts of residues) ...
... Catalytic site (functionally important residues – often very very specific) Hydrophobic core (tolerates most hydrobic residues, and polar uncharged residues) Hydrophilic Surface (tolerates almost all sorts of residues) ...
Center for Eukaryotic Structural Genomics (CESG)
... binding protein allowed expression of the full-length cyt b5 (fl-cytb5) as a fully soluble entity. Maintenance of the solubility in E. coli during the time course of expression was associated with high-level incorporation of protoporphyrin IX into the heme domain of the fusion protein. The fl-cytb5 ...
... binding protein allowed expression of the full-length cyt b5 (fl-cytb5) as a fully soluble entity. Maintenance of the solubility in E. coli during the time course of expression was associated with high-level incorporation of protoporphyrin IX into the heme domain of the fusion protein. The fl-cytb5 ...
Denaturation of proteins
... therefore, will lose their charge or become charged, depending on which way the pH is changed and by how much. That will eliminate some, perhaps many, of the ionic interactions that were necessary for maintenance of the folded shape of the protein. Finally, exposure of a protein to an organic solven ...
... therefore, will lose their charge or become charged, depending on which way the pH is changed and by how much. That will eliminate some, perhaps many, of the ionic interactions that were necessary for maintenance of the folded shape of the protein. Finally, exposure of a protein to an organic solven ...
Biochemistry H Silent Tea Party Name_______________ 1. What is
... makes up most of the cell membrane. 46. What is peptide bond? the bond between two amino acids 47. What is alpha helix? the spiral shape formed by some polypeptide chains as proteins configure into their final shape. This is an option for the secondary structure of a protein. 48. What is beta pleate ...
... makes up most of the cell membrane. 46. What is peptide bond? the bond between two amino acids 47. What is alpha helix? the spiral shape formed by some polypeptide chains as proteins configure into their final shape. This is an option for the secondary structure of a protein. 48. What is beta pleate ...
Protein folding

Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil.Each protein exists as an unfolded polypeptide or random coil when translated from a sequence of mRNA to a linear chain of amino acids. This polypeptide lacks any stable (long-lasting) three-dimensional structure (the left hand side of the first figure). Amino acids interact with each other to produce a well-defined three-dimensional structure, the folded protein (the right hand side of the figure), known as the native state. The resulting three-dimensional structure is determined by the amino acid sequence (Anfinsen's dogma). Experiments beginning in the 1980s indicate the codon for an amino acid can also influence protein structure.The correct three-dimensional structure is essential to function, although some parts of functional proteins may remain unfolded, so that protein dynamics is important. Failure to fold into native structure generally produces inactive proteins, but in some instances misfolded proteins have modified or toxic functionality. Several neurodegenerative and other diseases are believed to result from the accumulation of amyloid fibrils formed by misfolded proteins. Many allergies are caused by incorrect folding of some proteins, because the immune system does not produce antibodies for certain protein structures.