Huang, David, Center for Structural Biochemistry
... Structure Determination – The electron density data was used to determine the structure of the proteins in complex with the ligands using the COOT software. Analysis – The specific hydrogen bonds or hydrophobic interactions around the ligand binding pocket were analyzed after the structure had b ...
... Structure Determination – The electron density data was used to determine the structure of the proteins in complex with the ligands using the COOT software. Analysis – The specific hydrogen bonds or hydrophobic interactions around the ligand binding pocket were analyzed after the structure had b ...
Protein PPT Editted
... fat to form lipoproteins, transport of iron and other nutrients and oxygen in the blood Provides energy as a last resort if the body can’t get energy from carbs and fat or if there is too much protein in the diet Protein provides 4 calories per gram ...
... fat to form lipoproteins, transport of iron and other nutrients and oxygen in the blood Provides energy as a last resort if the body can’t get energy from carbs and fat or if there is too much protein in the diet Protein provides 4 calories per gram ...
Nick Grishin "Evolutionary Classification of Protein Domains
... such as spatial structures and functions. Homology is frequently obscured by sequence divergence, spatial structure changes and resemblance between unrelated 3D structures. We have developed a hierarchical evolutionary classification of all proteins with experimentally determined spatial structures. ...
... such as spatial structures and functions. Homology is frequently obscured by sequence divergence, spatial structure changes and resemblance between unrelated 3D structures. We have developed a hierarchical evolutionary classification of all proteins with experimentally determined spatial structures. ...
Sports Fitness
... When you eat foods that contain protein, you break down the protein in food into basic units, called amino acids .The amino acids then can be reused to make the proteins your body needs to maintain muscles, bones, blood, and body organs. ...
... When you eat foods that contain protein, you break down the protein in food into basic units, called amino acids .The amino acids then can be reused to make the proteins your body needs to maintain muscles, bones, blood, and body organs. ...
C h e m g u id e –... PROTEINS: STRUCTURE
... g) Proteins are described in terms of their primary, secondary and tertiary structures (and quaternary, but we aren’t concerned with that at this level). What level of structure are we talking about in the diagrams above? 3. The overall shape of a protein, the tertiary structure (if you said that in ...
... g) Proteins are described in terms of their primary, secondary and tertiary structures (and quaternary, but we aren’t concerned with that at this level). What level of structure are we talking about in the diagrams above? 3. The overall shape of a protein, the tertiary structure (if you said that in ...
FUNCTIONS OF PROTEINS IN THE BODY FUNCTIONS OF
... as well as in providing us an invaluable source of energy. In the absence of protein, the body would simply shut down. PROTEIN AS ENZYMES Proteins are responsible for almost every chemical reaction that takes place in the body. These reactions are facilitated by enzymes, which are actually protein c ...
... as well as in providing us an invaluable source of energy. In the absence of protein, the body would simply shut down. PROTEIN AS ENZYMES Proteins are responsible for almost every chemical reaction that takes place in the body. These reactions are facilitated by enzymes, which are actually protein c ...
ppt - Scientific Data Analysis Lab
... Disordered regions (DRs) are entire proteins or regions of proteins which lack a fixed tertiary structure, essentially being partially or fully unfolded. Such disordered regions have been shown to be involved in a variety of functions, including DNA recognition, modulation of specificity/affinity of ...
... Disordered regions (DRs) are entire proteins or regions of proteins which lack a fixed tertiary structure, essentially being partially or fully unfolded. Such disordered regions have been shown to be involved in a variety of functions, including DNA recognition, modulation of specificity/affinity of ...
Proteins - Northwest ISD Moodle
... make up a protein. - the interactions of the R groups on each amino acid cause the molecule to bend and fold – different arrangements create different shapes - as a result- the order of amino acids determines the shape of the protein - shape determines function - changing a single amino acid can cha ...
... make up a protein. - the interactions of the R groups on each amino acid cause the molecule to bend and fold – different arrangements create different shapes - as a result- the order of amino acids determines the shape of the protein - shape determines function - changing a single amino acid can cha ...
Recombinant human BRD9 protein (Active)
... The application notes include recommended starting dilutions; optimal dilutions/concentrations should be determined by the end user. ...
... The application notes include recommended starting dilutions; optimal dilutions/concentrations should be determined by the end user. ...
BNFO 602 Lecture 1 - New Jersey Institute of Technology
... twenty different amino acids that chain in different ways to form different proteins. For example, FLLVALCCRFGH (this is how we could store it in a file) This sequence of amino acids folds to form a 3-D structure ...
... twenty different amino acids that chain in different ways to form different proteins. For example, FLLVALCCRFGH (this is how we could store it in a file) This sequence of amino acids folds to form a 3-D structure ...
Symmetry
... Proteins are chiral objects, and cannot be mirror-inverted while remaining the same. Their mirror reflection is different. Thus, many of these arrangements are actually precluded. In fact, proteins may only adopt 65 of the 230 possible 3D space groups. Many of these are observed when we crystallize ...
... Proteins are chiral objects, and cannot be mirror-inverted while remaining the same. Their mirror reflection is different. Thus, many of these arrangements are actually precluded. In fact, proteins may only adopt 65 of the 230 possible 3D space groups. Many of these are observed when we crystallize ...
Bioinformatics for biomedicine Protein domains and 3D structure
... • Is crystal state relevant? • Short answer: Yes – High solvent content; similar to cell plasma – Comparisons indicate OK ...
... • Is crystal state relevant? • Short answer: Yes – High solvent content; similar to cell plasma – Comparisons indicate OK ...
defend your answer in 1
... I. Decrease energy level a with no effect on energy levels b or c II. Decrease energy levels a and c III. Decrease energy level a and increase c IV. Increase c while a and b remain the same ...
... I. Decrease energy level a with no effect on energy levels b or c II. Decrease energy levels a and c III. Decrease energy level a and increase c IV. Increase c while a and b remain the same ...
Early states during protein folding - The Astbury Centre for Structural
... determine structural and dynamic information, confirming the three-helical nature of the intermediate at the residue-specific level and revealing that whilst the intermediate remains a compact species, it is much more conformationally dynamic than the native state of the wild type protein. Using mo ...
... determine structural and dynamic information, confirming the three-helical nature of the intermediate at the residue-specific level and revealing that whilst the intermediate remains a compact species, it is much more conformationally dynamic than the native state of the wild type protein. Using mo ...
Why Are McDonalds ingredients fake
... been taking protein as early as the 50’s but they didn’t take highly processed product and they took different amounts. The amount an average person needs is 1 gram per 100 pounds of body weight (for instance if you weighed 200 pounds the minimum amount of protein you would need to take in would be ...
... been taking protein as early as the 50’s but they didn’t take highly processed product and they took different amounts. The amount an average person needs is 1 gram per 100 pounds of body weight (for instance if you weighed 200 pounds the minimum amount of protein you would need to take in would be ...
Protein: A polymer of amino acids Amino Acid Structure
... – Coiled or folded shape held together by hydrogen bonds ...
... – Coiled or folded shape held together by hydrogen bonds ...
Biomolecules in water and water in biomolecules
... theory has demonstrated its amazing capability of “predicting” the process from the frist principle. [1] However, what we have investigated so far is an entirely equilibrium process both in protein conformation and solvation. Recently, we have started to incorporate the conformational fluctuation of ...
... theory has demonstrated its amazing capability of “predicting” the process from the frist principle. [1] However, what we have investigated so far is an entirely equilibrium process both in protein conformation and solvation. Recently, we have started to incorporate the conformational fluctuation of ...
Protein Folding, Shape, and Function Activity Instructions
... proteins and other structures at the molecular level. In this activity you will explore the structure of proteins and the chemical interactions that drive each protein to fold into its specific structure. Each protein is made of a specific sequence of amino acids. There are 20 amino acids involved i ...
... proteins and other structures at the molecular level. In this activity you will explore the structure of proteins and the chemical interactions that drive each protein to fold into its specific structure. Each protein is made of a specific sequence of amino acids. There are 20 amino acids involved i ...
1 Glycosylation and Protein Folding I. Introduction. As a translocated
... I. Introduction. As a translocated polypeptide emerges into the lumen of the ER, it is generally processed in three ways: 1) its signal sequence is cleaved by signal peptidase; 2) it is glycosylated; and 3) it must be helped to fold into the correct conformation. II. Signal peptidase. Cleavage of th ...
... I. Introduction. As a translocated polypeptide emerges into the lumen of the ER, it is generally processed in three ways: 1) its signal sequence is cleaved by signal peptidase; 2) it is glycosylated; and 3) it must be helped to fold into the correct conformation. II. Signal peptidase. Cleavage of th ...
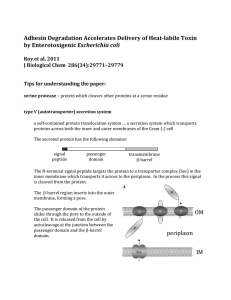
Paper background for Students
... short domain from the myc gene, followed by a region encoding 6 consecutive histidines The resulting fusion protein contains three domains: a. EtpA b. 10 amino acids of the myc protein sequence (a protein “tag) c. 6 histidine residues (a protein “tag”) This is useful because the protein can be purif ...
... short domain from the myc gene, followed by a region encoding 6 consecutive histidines The resulting fusion protein contains three domains: a. EtpA b. 10 amino acids of the myc protein sequence (a protein “tag) c. 6 histidine residues (a protein “tag”) This is useful because the protein can be purif ...
Recombinant human ADRB2 + GsalphaL fusion protein
... activation. ADRB2 binds epinephrine with an approximately 30-fold greater affinity than it does norepinephrine. Guanine nucleotide-binding proteins (G proteins) are involved as modulators or transducers in various transmembrane signaling systems. The Gs protein is involved in hormonal regulation of ...
... activation. ADRB2 binds epinephrine with an approximately 30-fold greater affinity than it does norepinephrine. Guanine nucleotide-binding proteins (G proteins) are involved as modulators or transducers in various transmembrane signaling systems. The Gs protein is involved in hormonal regulation of ...
Protein folding

Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil.Each protein exists as an unfolded polypeptide or random coil when translated from a sequence of mRNA to a linear chain of amino acids. This polypeptide lacks any stable (long-lasting) three-dimensional structure (the left hand side of the first figure). Amino acids interact with each other to produce a well-defined three-dimensional structure, the folded protein (the right hand side of the figure), known as the native state. The resulting three-dimensional structure is determined by the amino acid sequence (Anfinsen's dogma). Experiments beginning in the 1980s indicate the codon for an amino acid can also influence protein structure.The correct three-dimensional structure is essential to function, although some parts of functional proteins may remain unfolded, so that protein dynamics is important. Failure to fold into native structure generally produces inactive proteins, but in some instances misfolded proteins have modified or toxic functionality. Several neurodegenerative and other diseases are believed to result from the accumulation of amyloid fibrils formed by misfolded proteins. Many allergies are caused by incorrect folding of some proteins, because the immune system does not produce antibodies for certain protein structures.