2.5-Inorganic-Chemistry - Urban Moonshine Herb School
... that many of the chemical reactions necessary to maintain life require a very specific, finely tuned pH in order to function properly. This can be very helpful in assessing and treating imbalanced conditions: for example, a person may have an internal pH that is too low, or too acidic, from overeati ...
... that many of the chemical reactions necessary to maintain life require a very specific, finely tuned pH in order to function properly. This can be very helpful in assessing and treating imbalanced conditions: for example, a person may have an internal pH that is too low, or too acidic, from overeati ...
Chemical Equations
... (aka Oxidation Number) Hypothetical charge use to indicate the degree of oxidation (loss of electrons) Rules in assigning oxidation states: 1) The oxidation state of a free element is zero (0). ex. O2 (g), Ag (s) 2) The oxidation state of a monatomic ion is equal to its ionic charge. (ex. Na+, Cl-3) ...
... (aka Oxidation Number) Hypothetical charge use to indicate the degree of oxidation (loss of electrons) Rules in assigning oxidation states: 1) The oxidation state of a free element is zero (0). ex. O2 (g), Ag (s) 2) The oxidation state of a monatomic ion is equal to its ionic charge. (ex. Na+, Cl-3) ...
BIOLOGY 110
... How many different amino acids are there? What makes one amino acid different from another? What type of reaction is used to string A.A.s into proteins? What is the name applied to a covalent bond that is formed between two A.A.s in a protein? 5. Characterize the difference between primary, secondar ...
... How many different amino acids are there? What makes one amino acid different from another? What type of reaction is used to string A.A.s into proteins? What is the name applied to a covalent bond that is formed between two A.A.s in a protein? 5. Characterize the difference between primary, secondar ...
Grade 11 Chemistry E.. - hrsbstaff.ednet.ns.ca
... tell what type of reaction it is. a) lead (II) nitrate and sodium iodide react to make lead iodide and sodium nitrate. b) calcium carbonate when you heat it produces calcium oxide and carbon dioxide. c) propane reacts with oxygen to produce carbon dioxide and water d) copper metal and silver nitrate ...
... tell what type of reaction it is. a) lead (II) nitrate and sodium iodide react to make lead iodide and sodium nitrate. b) calcium carbonate when you heat it produces calcium oxide and carbon dioxide. c) propane reacts with oxygen to produce carbon dioxide and water d) copper metal and silver nitrate ...
Amino acids
... - myoglobin is 90% saturated (gives back only 10%) - hemoglobin is 50% saturated (gives back 50%) ...
... - myoglobin is 90% saturated (gives back only 10%) - hemoglobin is 50% saturated (gives back 50%) ...
A mutant defective in enzyme
... (a)Fructose (b)Galactose (c)Ribose (d)Sucrose 2. The enzyme that joins DNA fragments cut by restriction enzymes is called: (a)Primase (b)Polymerase (c)Ligase (d)DNA phosphorylase 3. The two features of the tRNA molecule involved in converting the triplet codon to an amino acid are: (a)in the anticod ...
... (a)Fructose (b)Galactose (c)Ribose (d)Sucrose 2. The enzyme that joins DNA fragments cut by restriction enzymes is called: (a)Primase (b)Polymerase (c)Ligase (d)DNA phosphorylase 3. The two features of the tRNA molecule involved in converting the triplet codon to an amino acid are: (a)in the anticod ...
[Fe 4 S 4 Cys 4 ] 1
... • In (deoxy)hemoglobin, Fe(II) is 5-coordinate • Must avoid oxidation to Fe(III) (Met-hemoglobin) • Neutral His ligand: His-Fe(II)-porphyrin is uncharged: Favourable • P450: Catalyses hydroxylation of hydrophobic substrates. Also 5-coordinate • 1 axial Cys thiolate ligand (negatively charged): Resti ...
... • In (deoxy)hemoglobin, Fe(II) is 5-coordinate • Must avoid oxidation to Fe(III) (Met-hemoglobin) • Neutral His ligand: His-Fe(II)-porphyrin is uncharged: Favourable • P450: Catalyses hydroxylation of hydrophobic substrates. Also 5-coordinate • 1 axial Cys thiolate ligand (negatively charged): Resti ...
Lecture Resource ()
... In each of these transformations, one of the bonds to the a-carbon of the amino acid substrate is broken in the first step of the reaction ...
... In each of these transformations, one of the bonds to the a-carbon of the amino acid substrate is broken in the first step of the reaction ...
Chapter 6, Section 3
... 1. Carbon forms bonds easily because it has 4 valence electrons. 2. Carbon atoms can bond to other carbon atoms, forming chains that are almost unlimited in length. 3. All living things contain carbon (C), hydrogen (H), oxygen (O), nitrogen (N), and phosphorous (P). ...
... 1. Carbon forms bonds easily because it has 4 valence electrons. 2. Carbon atoms can bond to other carbon atoms, forming chains that are almost unlimited in length. 3. All living things contain carbon (C), hydrogen (H), oxygen (O), nitrogen (N), and phosphorous (P). ...
Activities 3
... polymerization. These connecting bonds are called phosphodiester bonds. a. This is true b. This is false ...
... polymerization. These connecting bonds are called phosphodiester bonds. a. This is true b. This is false ...
Biochemistry Test Review Guide
... c. Where in the atom are they located? 2. What does the atomic number on the periodic table tell us? 3. How is the mass number found? What does it tell us? 4. Define and give one example of each: a. Atom (definition only) c. Molecule b. Element d. Compound 5. Given isotope notation to the right, fin ...
... c. Where in the atom are they located? 2. What does the atomic number on the periodic table tell us? 3. How is the mass number found? What does it tell us? 4. Define and give one example of each: a. Atom (definition only) c. Molecule b. Element d. Compound 5. Given isotope notation to the right, fin ...
Coordination compounds in nature
... oxygen. Hemoglobin is constituted of four heme units. Hemoglobin transports oxygen from the lungs to the cells of the body and there it transfers the oxygen to myoglobin which contains only one heme unit. The myoglobin makes the oxygen available to the respiratory reactions of the cell. ...
... oxygen. Hemoglobin is constituted of four heme units. Hemoglobin transports oxygen from the lungs to the cells of the body and there it transfers the oxygen to myoglobin which contains only one heme unit. The myoglobin makes the oxygen available to the respiratory reactions of the cell. ...
LESSON 2 - ASSIGNMENT 1. Differentiate between a fat and an oil
... following statements must then be true for the molecule (select all that are correct); a) b) c) d) e) f) g) ...
... following statements must then be true for the molecule (select all that are correct); a) b) c) d) e) f) g) ...
Chemical Bond - Cobb Learning
... positive and negative oxidation numbers is zero. The Crisscross Method or Swap N’ Drop Method can also work. 4) All compounds are neutral so the oxidation numbers should combine in ratios that will add up to zero. The number of ions combining in the compound will be written as subscripts in the fina ...
... positive and negative oxidation numbers is zero. The Crisscross Method or Swap N’ Drop Method can also work. 4) All compounds are neutral so the oxidation numbers should combine in ratios that will add up to zero. The number of ions combining in the compound will be written as subscripts in the fina ...
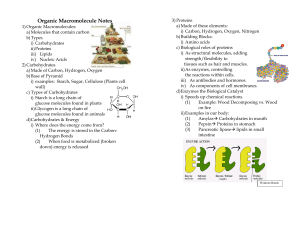
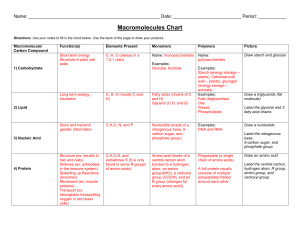
Organic Macromolecule Notes
... a) Made of these elements: i) Carbon, Hydrogen, Oxygen, Nitrogen b) Building Blocks: i) Amino acids c) Biological roles of proteins i) As structural molecules, adding strength/flexibility to tissues such as hair and muscles. ii) As enzymes, controlling the reactions within cells. iii) As antibodies ...
... a) Made of these elements: i) Carbon, Hydrogen, Oxygen, Nitrogen b) Building Blocks: i) Amino acids c) Biological roles of proteins i) As structural molecules, adding strength/flexibility to tissues such as hair and muscles. ii) As enzymes, controlling the reactions within cells. iii) As antibodies ...
Addition of the following reactions responsible for the synthesis of
... a. phosphatidate, old: C1836H3398O400P50, new: C1682H3116O413P50 b. phosphatidylglycerol, old: C1986H3748O500P50, new: C1832H3466O513P50 c. phosphatidylserine, old: C1986H3698N50O500P50, new: C1832H3416N50O513P50 d. CDP-diacylglycerol, old: C2286H3998N150O750P100, new: C2132H3716N150O763P100 e. card ...
... a. phosphatidate, old: C1836H3398O400P50, new: C1682H3116O413P50 b. phosphatidylglycerol, old: C1986H3748O500P50, new: C1832H3466O513P50 c. phosphatidylserine, old: C1986H3698N50O500P50, new: C1832H3416N50O513P50 d. CDP-diacylglycerol, old: C2286H3998N150O750P100, new: C2132H3716N150O763P100 e. card ...
Biochemistry
... between atoms Structure that results when atoms are joined together by covalent bonds is called a molecule ...
... between atoms Structure that results when atoms are joined together by covalent bonds is called a molecule ...
Bio 1000 Human Biology for Non-Majors
... Atoms have no overall charge; they are neutral. But we know that they have protons (positively charged particles) in the nucleus. Electrons are negative particles which cancel the protons charge. So in an atom there are equal numbers of electrons and protons. An ion is an atom which has lost or gain ...
... Atoms have no overall charge; they are neutral. But we know that they have protons (positively charged particles) in the nucleus. Electrons are negative particles which cancel the protons charge. So in an atom there are equal numbers of electrons and protons. An ion is an atom which has lost or gain ...
Chemical Compounds in Cells and in Our Food
... • Contain C, H, O, N and sometimes Sulfur • Found in many foods • In the cell, used as: -part of cell membranes -structures of organelles -muscles in the body ...
... • Contain C, H, O, N and sometimes Sulfur • Found in many foods • In the cell, used as: -part of cell membranes -structures of organelles -muscles in the body ...
Lecture 13
... by far the more stable oxidation state, and Tl(III)I3 is, for example, an unknown compound. Bi(V) is known in only one or two compounds of doubtful validity. The resistance of Hg metal to oxidation, and its existence as a liquid at room temperature, can be viewed as a manifestation of the inert pair ...
... by far the more stable oxidation state, and Tl(III)I3 is, for example, an unknown compound. Bi(V) is known in only one or two compounds of doubtful validity. The resistance of Hg metal to oxidation, and its existence as a liquid at room temperature, can be viewed as a manifestation of the inert pair ...
Water as a Brønsted acid or base
... between the Na+ ion and the H2O molecules. (b) If the metal–oxygen bond possesses significant covalent character, the first hydration shell can be reasonably represented showing oxygen-to-metal ion coordinate bonds; however, there is also an ionic contribution to the bonding interaction. ...
... between the Na+ ion and the H2O molecules. (b) If the metal–oxygen bond possesses significant covalent character, the first hydration shell can be reasonably represented showing oxygen-to-metal ion coordinate bonds; however, there is also an ionic contribution to the bonding interaction. ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.







![[Fe 4 S 4 Cys 4 ] 1](http://s1.studyres.com/store/data/008100934_1-bb8d7235eb07199e709035eea64be997-300x300.png)