Chemistry of Coordination Compounds
... while the purple compound only has 5 ammonia molecules in the coordinated compound. As shown in the ball-and-stick model, the chlorides serve as counter ions to the cobalt/ammonia coordiation complex in the orange compound, while one of the ammonia molecules is replaced by Cl in the purple compound. ...
... while the purple compound only has 5 ammonia molecules in the coordinated compound. As shown in the ball-and-stick model, the chlorides serve as counter ions to the cobalt/ammonia coordiation complex in the orange compound, while one of the ammonia molecules is replaced by Cl in the purple compound. ...
Biol 1441
... Two atoms are so unequal in their attraction for valence electrons that the more electronegative atom strips an electron completely away from its partner. Ion: a charged atom (or molecule) Cation: a positive ion Anion: a negative ion The transfer of an electron is not the formation of a bond; rather ...
... Two atoms are so unequal in their attraction for valence electrons that the more electronegative atom strips an electron completely away from its partner. Ion: a charged atom (or molecule) Cation: a positive ion Anion: a negative ion The transfer of an electron is not the formation of a bond; rather ...
Prentice hall Biology Worksheets
... Abase is a compound that produces OHions in solution. Basic, or alkaline, solutions contain lower concentrations of H+ ions than pure water and have pH values above 7. ...
... Abase is a compound that produces OHions in solution. Basic, or alkaline, solutions contain lower concentrations of H+ ions than pure water and have pH values above 7. ...
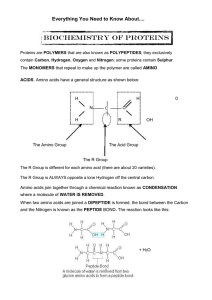
AS Biology - Everything Protein
... PRIMARY STRUCTURE is the AMINO ACID SEQUENCE; peptide bonds are present in this level of structure. SECONDARY STRUCTURE is how the primary structure folds for the first time. The two most common secondary structure folds are shown below: ...
... PRIMARY STRUCTURE is the AMINO ACID SEQUENCE; peptide bonds are present in this level of structure. SECONDARY STRUCTURE is how the primary structure folds for the first time. The two most common secondary structure folds are shown below: ...
Reading Guide: Pratt and Cornely, Chapter 5.1 1. Compare and
... Reading Guide: Pratt and Cornely, Chapter 5.1 1. Compare and contrast the roles of myoglobin and hemoglobin. 2. Describe the major structural features of myoglobin and how they are similar/different than hemoglobin. 3. Draw a simple schematic of the ligands that bind to the iron ion found in myoglob ...
... Reading Guide: Pratt and Cornely, Chapter 5.1 1. Compare and contrast the roles of myoglobin and hemoglobin. 2. Describe the major structural features of myoglobin and how they are similar/different than hemoglobin. 3. Draw a simple schematic of the ligands that bind to the iron ion found in myoglob ...
9F Reactivity - Parrs Wood High School
... These substances contain hydrogen and carbon only. They burn in a plentiful supply of air to form carbon dioxide and water: hydrocarbon + oxygen → carbon dioxide + water The test for oxygen is that it relights a glowing splint. An input of energy from a flame or spark is needed to start the combusti ...
... These substances contain hydrogen and carbon only. They burn in a plentiful supply of air to form carbon dioxide and water: hydrocarbon + oxygen → carbon dioxide + water The test for oxygen is that it relights a glowing splint. An input of energy from a flame or spark is needed to start the combusti ...
CHAPTER 2 OBJECTIVE EXERCISE
... Compare and contrast the major divisions (types of chemical reactions) of metabolism, in terms of a general descriptive sentence, additional descriptive terms, how energy is involved, whether bonds are formed or broken, and how water is involved. Also write a chemical reaction for each and give an e ...
... Compare and contrast the major divisions (types of chemical reactions) of metabolism, in terms of a general descriptive sentence, additional descriptive terms, how energy is involved, whether bonds are formed or broken, and how water is involved. Also write a chemical reaction for each and give an e ...
Photosynthesis “Carbon Fixation” λ Energy H20 O2 water oxidized
... reactions that are energetically favorable reactions to those that are energetically unfavorable ...
... reactions that are energetically favorable reactions to those that are energetically unfavorable ...
2009-10 Chemistry 1st Semester Final Exam Topics and Review
... e. One of the problems with space travel is the building up of carbon dioxide produced by the astronauts. The typical procedure is to react the carbon dioxide with lithium hydroxide to form lithium carbonate and liquid water. 36. Write and balance a chemical equation for this reaction. a. Aluminum m ...
... e. One of the problems with space travel is the building up of carbon dioxide produced by the astronauts. The typical procedure is to react the carbon dioxide with lithium hydroxide to form lithium carbonate and liquid water. 36. Write and balance a chemical equation for this reaction. a. Aluminum m ...
Unit 1 Page 1 Unit Vocabulary Terms Carbohydrate
... ● Carbohydrate - A group that includes sugar and starch that is used for energy or structure; can be small molecules (monosaccharides or disaccharides) or large molecules (polysaccharides such as starch and cellulose.) ● Proteins - A three-dimensional biological macromolecule constructed from a set ...
... ● Carbohydrate - A group that includes sugar and starch that is used for energy or structure; can be small molecules (monosaccharides or disaccharides) or large molecules (polysaccharides such as starch and cellulose.) ● Proteins - A three-dimensional biological macromolecule constructed from a set ...
energy currency for cell - Hermantown Community Schools
... R group makes the amino acids different from each other. • The R groups between the different amino acids help create the proteins shape. • Folds and bonds form creating distinct protein shapes ...
... R group makes the amino acids different from each other. • The R groups between the different amino acids help create the proteins shape. • Folds and bonds form creating distinct protein shapes ...
Re-typed from The Ultimate Chemical Equations Handbook by
... 1. A binary molecule is formed when two nonmetals or metalloids combine. Electrons are shared so the bonding involved is known as _________________________ bonding. 2. Sometimes these compounds have generic or common names (water) and they also have systemic names (dihydrogen monoxide). The common n ...
... 1. A binary molecule is formed when two nonmetals or metalloids combine. Electrons are shared so the bonding involved is known as _________________________ bonding. 2. Sometimes these compounds have generic or common names (water) and they also have systemic names (dihydrogen monoxide). The common n ...
Symmetry
... two forms are near-identical (both consisting of 374 amino acids), as there are only six residues which are different. None of these six occur in the interface region between the two. There are three possible combinations of the subunits: EE, SS and ES; these are therefore isozymes (or isoenzymes). ...
... two forms are near-identical (both consisting of 374 amino acids), as there are only six residues which are different. None of these six occur in the interface region between the two. There are three possible combinations of the subunits: EE, SS and ES; these are therefore isozymes (or isoenzymes). ...
Chem*3560 Lecture 1: Structure and Function in Biochemistry
... slows down catalysis of other substrates may be called an inhibitor. A ligand that binds to an enzyme and does not participates in reaction, but regualtes catalytic activity may be called an effector. The term ligand is also applied to small molecules that bind to metal ions to form complex ions, e. ...
... slows down catalysis of other substrates may be called an inhibitor. A ligand that binds to an enzyme and does not participates in reaction, but regualtes catalytic activity may be called an effector. The term ligand is also applied to small molecules that bind to metal ions to form complex ions, e. ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.