Bio-Chem Notes
... • Only contain the elements carbon, hydrogen, and oxygen in a specific ratio of 1:2:1 Example: formula for glucose is C6H12O6 ...
... • Only contain the elements carbon, hydrogen, and oxygen in a specific ratio of 1:2:1 Example: formula for glucose is C6H12O6 ...
THE CHEMISTRY OF LIFE – CH
... Hydrogen bonds are important to organisms because they hold many biomolecules (proteins, carbohydrates) together. Organic Compounds (compounds made with carbon): are macromolecules (or polymers) – large molecules made when smaller molecules bond together to form chains. ...
... Hydrogen bonds are important to organisms because they hold many biomolecules (proteins, carbohydrates) together. Organic Compounds (compounds made with carbon): are macromolecules (or polymers) – large molecules made when smaller molecules bond together to form chains. ...
Ch 12 Electrolysis in water - Copley
... This is the broadest definition. An acid is an electron pair acceptor. A base is an electron pair donor. It is easy to remember that Lewis acids and bases are electron pair donor or acceptors because Lewis is famous for electron dot ...
... This is the broadest definition. An acid is an electron pair acceptor. A base is an electron pair donor. It is easy to remember that Lewis acids and bases are electron pair donor or acceptors because Lewis is famous for electron dot ...
Chemical Formulas
... A neutral nitrogen atom has 7 electrons. Two of those electrons fill up the innermost energy level. a. How many electrons does nitrogen atom have in the second energy level? b. Is nitrogen more likely to give away the electrons in its second energy level, or to take in other electrons in order to ha ...
... A neutral nitrogen atom has 7 electrons. Two of those electrons fill up the innermost energy level. a. How many electrons does nitrogen atom have in the second energy level? b. Is nitrogen more likely to give away the electrons in its second energy level, or to take in other electrons in order to ha ...
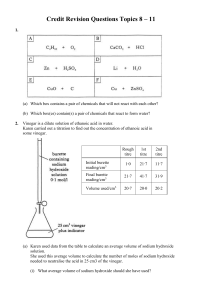
Credit Revision Questions Topics 8 – 11 1. (a) Which box contains a
... 3Ca2+(aq) + 6C1-(aq) + 6Na+(aq) + 2PO43-(aq) → 6Na+(aq) + 6C1-(aq) + (Ca2+)3(PO43-)2(s) Circle the spectator ions in the above equation. ...
... 3Ca2+(aq) + 6C1-(aq) + 6Na+(aq) + 2PO43-(aq) → 6Na+(aq) + 6C1-(aq) + (Ca2+)3(PO43-)2(s) Circle the spectator ions in the above equation. ...
e-nomenclature-of-coordination-compounds-take-home-2
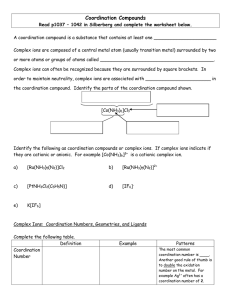
... A coordination compound is a substance that contains at least one ______________________ Complex ions are composed of a central metal atom (usually transition metal) surrounded by two or more atoms or groups of atoms called ________________________________________. Complex ions can often be recogniz ...
... A coordination compound is a substance that contains at least one ______________________ Complex ions are composed of a central metal atom (usually transition metal) surrounded by two or more atoms or groups of atoms called ________________________________________. Complex ions can often be recogniz ...
Chapter 2 Study Guide
... 36. Reactant 37. Product 38. Activation Energy 39. Catalyst 40. Enzyme 41. Substrate ...
... 36. Reactant 37. Product 38. Activation Energy 39. Catalyst 40. Enzyme 41. Substrate ...
Chemistry Unit Review
... 4. Describe the difference between a physical change and a chemical change. ...
... 4. Describe the difference between a physical change and a chemical change. ...
CH 2.3-Carbon Compounds
... - Have an amino group on one end and a carboxyl group on the other - Have 20 different types ...
... - Have an amino group on one end and a carboxyl group on the other - Have 20 different types ...
chapter 18 - rci.rutgers.edu
... Amino acid catabolism starts with transamination. Amino groups are transferred to alpha-ketoglutarate by specific aminotransferase enzymes (656). The resulting glutamate is then oxidatively deaminated by glutamate DH which can use either NAD+ or NADP+. The ammonia produced is generally incorporated ...
... Amino acid catabolism starts with transamination. Amino groups are transferred to alpha-ketoglutarate by specific aminotransferase enzymes (656). The resulting glutamate is then oxidatively deaminated by glutamate DH which can use either NAD+ or NADP+. The ammonia produced is generally incorporated ...
File - Principles of Biology 103
... 10. What is the building block of carbohydrates? A. Monosaccharides 11. The nonadjacent regions that form to create specific domains is termed: C. Tertiary structure 12. A nucleotide contains: A. A five carbon ring bonded to a nitrogen base and a phosphate group(s) 13. The breakdown of large molecul ...
... 10. What is the building block of carbohydrates? A. Monosaccharides 11. The nonadjacent regions that form to create specific domains is termed: C. Tertiary structure 12. A nucleotide contains: A. A five carbon ring bonded to a nitrogen base and a phosphate group(s) 13. The breakdown of large molecul ...
Metal Ion Transport and Storage
... • Charged Ions must pass through a Hydrophobic Membrane – Neutral gases (O2, CO2) and low charge density ions (anions) can move directly through the membrane – High charge density cations require help • Once inside the cell, metal ions must be transported to the location of their use, then released ...
... • Charged Ions must pass through a Hydrophobic Membrane – Neutral gases (O2, CO2) and low charge density ions (anions) can move directly through the membrane – High charge density cations require help • Once inside the cell, metal ions must be transported to the location of their use, then released ...
Lab 11
... Transition metals are positively charged and will readily accept electrons. Ligand molecules contain atoms with non-bonding electrons, which can be donated to the electron deficient metal ion in order to form a complex ion. This can be viewed as Lewis acid – base chemistry because of the electron in ...
... Transition metals are positively charged and will readily accept electrons. Ligand molecules contain atoms with non-bonding electrons, which can be donated to the electron deficient metal ion in order to form a complex ion. This can be viewed as Lewis acid – base chemistry because of the electron in ...
The Representative Elements
... • Have metallic physical and chemical properties. • In forming ionic compounds with nonmetals, the transition metals exhibit several typical characteristics: • More than one oxidation state is usually found • Cations are often complex ions • Most compounds are colored • Most compounds have paramagn ...
... • Have metallic physical and chemical properties. • In forming ionic compounds with nonmetals, the transition metals exhibit several typical characteristics: • More than one oxidation state is usually found • Cations are often complex ions • Most compounds are colored • Most compounds have paramagn ...
04 Biochemistry
... them up. • Octet rule = an atom in 2nd energy level always likes to have 8 e- on the outermost energy level. • When bonds form between two atoms, only the unpaired valence e- from the two atoms pair up. ...
... them up. • Octet rule = an atom in 2nd energy level always likes to have 8 e- on the outermost energy level. • When bonds form between two atoms, only the unpaired valence e- from the two atoms pair up. ...
04b Carbohydrates-student note
... an insoluble linear polysaccharide of D-glucose, used for support in plants joined by β 1-4 linkages its bonds are resistant to hydrolysis important in our diet as fiber Cellulose like polymer used in the exoskeletons of bugs and lobsters and the cell wall of some fungi ...
... an insoluble linear polysaccharide of D-glucose, used for support in plants joined by β 1-4 linkages its bonds are resistant to hydrolysis important in our diet as fiber Cellulose like polymer used in the exoskeletons of bugs and lobsters and the cell wall of some fungi ...
Protein Structure
... Lower His bonds covalently to iron(II) Oxygen coordinates to sixth site on iron and the upper His acts as a “gate” for the oxygen. ...
... Lower His bonds covalently to iron(II) Oxygen coordinates to sixth site on iron and the upper His acts as a “gate” for the oxygen. ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.