* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The Representative Elements
Survey
Document related concepts
Transcript
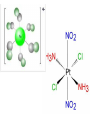
The Representative Elements Ch 18 & 19 Representative Elements • Their chemical properties are determined by their valence (s and p) electrons. • Properties are similar within a group, but first element in a group tends to act differently due to smaller size. • Most abundant element is oxygen, followed by silicon. • Most abundant metals are aluminum and iron, which are found as ores. Group 1 (1A) – Alkali Metals • Have Valence electron configuration ns1 • Will lose 1 electron to form M+ Ions • React vigorously with water to form M+ and OH- ions and hydrogen gas. • React with oxygen to form oxides. Lithium will form a regular oxide (Li2O) while sodium will form a peroxide (Na2O2). Potassium, rubidium and cesium form superoxides (MO2) Selected Reactions of the Alkali Metals Reaction Comment 2M + X2 2MX X2 = any halogen molecule 4Li + O2 2Li2O Excess oxygen 2Na + O2 Na2O2 M + O2 MO2 M = K, Rb or Cs 2M + S M2S 6Li + N2 2Li3N Li only 12M + P4 4M3P 2M + H2 2MH 2M + 2H2O 2MOH + H2 2M + 2H+ 2M+ + H2 Violent reaction! Hydrogen • Can form covalent compounds with other nonmetals. • Will form salts, hydrides, with very active metals (group 1A and 2A). • Hydride ion, H-, is a strong reducing agent. • Covalent hydrides form when hydrogen bonds with other nonmetals. • Metallic hydrides occur when hydrogen atoms migrate into transition metal crystals. Group 2 (2A) – Alkaline Earth Metals • Have valence electron configuration ns2 • Called Alkaline earth because of the basicity of their oxides. • React less vigorously with water than group 1. • Heavier alkaline earth metals form ionic nitrides and hydrides. • Hard water is caused by the presence of Ca+2 and Mg+2 ions. Selected Reactions of the Alkaline Earth Metals Reaction Comments M + X2 MX2 X2 = any halogen molecule 2M + O2 2MO Ba gives BaO2 as well M + S MS 3M + N2 M3N2 High temperatures 6M + P4 2M3P2 High temperatures M + H2 MH2 M = Ca, Sr or Ba; high temperatures; Mg also needs high pressure M + 2H2O M(OH)2 + H2 M = Ca, Sr or Ba M + 2H+ M+2 + H2 Be + 2OH- + 2H2O Be(OH)42- + H2 Group 13 (3A) • Have valence electron configuration ns2 np1 • Show increasing metallic character going down the group. • Boron forms covalent compounds with hydrides called boranes. These compounds are highly electron deficient and very reactive. • Aluminum has some covalent characteristics as do indium and gallium. • Thallium is completely metallic in character. Selected Reactions for Group 13 (3A) Elements Reactions Comments X2 = any halogen molecule; Tl gives TlX as well but no TlI3 2M + 3X2 2 MX3 High temperatures; Tl gives Tl2O as well 4M + 3O2 2M2O3 High temperatures; Tl gives Tl2S as well 2M + 3S M2S3 M = Al only 2M + N2 2MN 2M + 6H+ 2M + 2OH- 2M3+ M = Al, Ga or In; Tl gives Tl+ + 3H2 + 6H2O 2M(OH)4 + 3H2 - M = Al or Ga Group 14 (4A) • Have valence electron configuration ns2 np2 • Show a change from nonmetallic to metallic properties going down the group. • All elements in this group form covalent bonds with nonmetals. • MX4 compounds (except carbon)react with Lewis bases to form two additional covalent bonds. Selected Reactions of the Group 14 (4A) Elements Reactions Comments M + 2X2 MX4 X2 = any halogen molecule; M = Ge, or Sn; Pb gives PbX2 M + O2 MO2 M = Ge, or Sn; high temperatures; Pb gives PbO or Pb3O4 M + 2H+ M2+ + H2 M = Sn or Pb Group 15 (5A) • Have varied chemical properties. • All members except nitrogen form molecules with 5 covalent bonds. (Nitrogen has no d sublevel) • Nitrogen and Phosphorous are nonmetals and form 3- anions in salts with active metals. • Antimony and bismuth are metallic. However their 5+ cations tend to be molecular rather than ionic. Nitrogen • The strength of the triple bond in the N2 molecule is important both thermodynamically and kinetically as they decompose exothermically. • The nitrogen cycle is the process through which nitrogen is recycled through the environment. • Nitrogen forms a series of oxides in which it has an oxidation state ranging from 1 to 5. • Nitric acid is a strong acid which is important as a reducing agent. • Ammonia is the most important nitrogen hydride. • Has pyramidal molecules with polar bonds. • Hydrazine (N2H4) is a powerful reducing agent. Phosphorous • Exists in three elemental forms: white, black and red. • Phosphine (PH3) has a structure analogous to that of ammonia but with bond angles closer to 90o. • Forms two oxides with oxidation states of 3+ and 5+. Group 16 (6A) • Shows the usual tendency of increasing metallic properties going down the group. • None behave as typical metals • Achieve noble gas configurations by adding two electrons to form 2- anions. • Form covalent bonds with other nonmetals. • Oxygen exists in two elemental forms: O2 and O3. • Sulfur has two elemental forms, both of which contain stacks of S8 rings. • Sulfur also forms two oxides: SO2 and SO. • Sulfur forms a variety of compounds in which it has a +6, +4, +2, 0 or -2 oxidation state. Group 17 (7A) - Halogens • This group consist of all nonmetals. • Form hydrogen halides (HX) that behave as strong acids in water, except for hydrogen fluoride. • Oxyacids of the halogens become stronger as the number of oxygen atoms attached to the halogen increase. • Interhalogens are compounds of two different halogens • Halogen-carbon compounds are important industrially: examples are Teflon, PVC and the Freons. Group 18 (8A) – Noble Gases • Full valence shells ns2 np6 • Generally unreactive. • Krypton, xenon and radon will form compounds with the highly electronegative elements fluorine and oxygen. Transition Elements Ch 20 The last electron in transition metals occupy the d sublevel. Because these inner orbitals cannot participate as easily in bonding as the s and p orbitals the chemistry is not greatly affected by the gradual change in the increased number of electrons in the d orbital. • Have metallic physical and chemical properties. • In forming ionic compounds with nonmetals, the transition metals exhibit several typical characteristics: • More than one oxidation state is usually found • Cations are often complex ions • Most compounds are colored • Most compounds have paramagnetic properties • Transition metals form a variety of ions by losing one or more of their electrons. • The maximum possible oxidation state for a given transition element corresponds to the loss of all the s and d electrons. • Transition metals form coordination compounds which consist of complex ions and counter ions. • These coordination complexes are very important to biological chemistry. Naming Coordination Compounds • The cation is named before the anion • The ligands are named before the metal ion • An o is added to the root name of an anion in the ligand • Prefixes are used to denote the number of simple ligands • The oxidation state of the central metal ion is designated by a Roman numeral in parenthesis • If more than one ligand is present, name them alphabetically (prefixes do not count) • If complex ion has a negative charge, the suffix –ate is added to the name of the metal