CONTRIBUTORS TO THIS VOLUME A. A. Woolf
... dissolved substance expresses its preference between two liquids simultaneously present and available to the substance. This preference is determined by chemical and physical principles which are inviolable but in most instances are identified in a qualitative fashion only. Consequently the behavior ...
... dissolved substance expresses its preference between two liquids simultaneously present and available to the substance. This preference is determined by chemical and physical principles which are inviolable but in most instances are identified in a qualitative fashion only. Consequently the behavior ...
D. Bagchi, S. Chaudhuri, S. Sardar, S. Choudhury, N. Polley, P
... samples. It has to be noted that BQ attached curcumin structure might not be similar to that of metallo curcumin complexes but excited state electron transfer timescale is similar due to proximity between two entities in both the cases. Thus, the shorter timescale in presence of metal ions can be ra ...
... samples. It has to be noted that BQ attached curcumin structure might not be similar to that of metallo curcumin complexes but excited state electron transfer timescale is similar due to proximity between two entities in both the cases. Thus, the shorter timescale in presence of metal ions can be ra ...
as a PDF
... eight elements within its compass (S.A. Cotton and F.A. Hart, The Heavy Transition Elements, Macmillan, 1975). This volume shares the same aim of covering the descriptive chemistry of silver, gold and the six platinum metals in some detail at a level suitable for advanced undergraduate and postgradu ...
... eight elements within its compass (S.A. Cotton and F.A. Hart, The Heavy Transition Elements, Macmillan, 1975). This volume shares the same aim of covering the descriptive chemistry of silver, gold and the six platinum metals in some detail at a level suitable for advanced undergraduate and postgradu ...
4134gdisk doc..4134gdisk chapter .. Page501
... transfer to RuNO as a rate determining step.81 Tris(bipyridyl)ruthenium(II) modifies mono-, di- and tri-nuclear manganese complexes as electron-transfer models for photosynthetic processes.82 Rate constants for the oxidation of nucleotides and DNA by [Ru(terpy)(bpy)O]2+ type complexes have been foun ...
... transfer to RuNO as a rate determining step.81 Tris(bipyridyl)ruthenium(II) modifies mono-, di- and tri-nuclear manganese complexes as electron-transfer models for photosynthetic processes.82 Rate constants for the oxidation of nucleotides and DNA by [Ru(terpy)(bpy)O]2+ type complexes have been foun ...
الشريحة 1
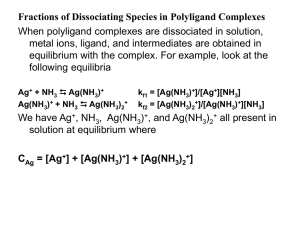
... since b0 is a function of Ag+. We use the equilibrium constants of each step: CAg = [Ag+] + [Ag(NH3)+] + [Ag(NH3)2+] kf1 = [Ag(NH3)+]/[Ag+][NH3] [Ag(NH3)+] = kf1 [Ag+][NH3] Kf1 x kf2 = [Ag(NH3)2+]/[Ag+][NH3]2 [Ag(NH3)2+] = Kf1 x kf2 [Ag+][NH3]2 Substitution in the CAg relation gives: CAg = [Ag+] + k ...
... since b0 is a function of Ag+. We use the equilibrium constants of each step: CAg = [Ag+] + [Ag(NH3)+] + [Ag(NH3)2+] kf1 = [Ag(NH3)+]/[Ag+][NH3] [Ag(NH3)+] = kf1 [Ag+][NH3] Kf1 x kf2 = [Ag(NH3)2+]/[Ag+][NH3]2 [Ag(NH3)2+] = Kf1 x kf2 [Ag+][NH3]2 Substitution in the CAg relation gives: CAg = [Ag+] + k ...
Linkage Isomerization Reactions*
... Production of metastable linkage isomers depends on the intrinsic labile/inert characteristics of the metal complex, in addition to the metal-ligand affinities and stereochemical factors. For instance, metal complexes, exhibiting low spin d5 and d6 configurations are substitution inert. Although thi ...
... Production of metastable linkage isomers depends on the intrinsic labile/inert characteristics of the metal complex, in addition to the metal-ligand affinities and stereochemical factors. For instance, metal complexes, exhibiting low spin d5 and d6 configurations are substitution inert. Although thi ...
PREPARATION OF COORDINATION COMPOUNDS Coordination
... (Cis-dinitrobis(ethylenediamine)cobalt III) Other preparative methods involving substitution reactions in non-aqueous media are: i. ...
... (Cis-dinitrobis(ethylenediamine)cobalt III) Other preparative methods involving substitution reactions in non-aqueous media are: i. ...
Spectroscopic Comparisons of the pH Dependencies of Fe
... coordinated solvent is believed to be H2O in (reduced) Fe2+SOD and to donate a proton to substrate upon oxidation of Fe to become OH- in (oxidized) Fe3+-SOD (6). The amino acids immediately surrounding the active site are especially highly conserved among Fe- and Mn-SODs. In particular, His 30 and T ...
... coordinated solvent is believed to be H2O in (reduced) Fe2+SOD and to donate a proton to substrate upon oxidation of Fe to become OH- in (oxidized) Fe3+-SOD (6). The amino acids immediately surrounding the active site are especially highly conserved among Fe- and Mn-SODs. In particular, His 30 and T ...
+ H 2 O(l) - Knockhardy
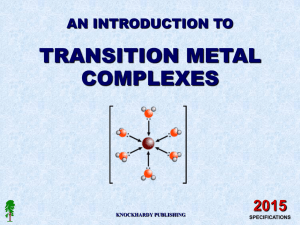
... aqueous metal ions attract water molecules many have six water molecules surrounding them these are known as hexaaqua ions they are octahedral in shape water acts as a Lewis Base – a lone pair donor water forms a co-ordinate bond to the metal ion metal ions accept the lone pair - Lewis Acids ...
... aqueous metal ions attract water molecules many have six water molecules surrounding them these are known as hexaaqua ions they are octahedral in shape water acts as a Lewis Base – a lone pair donor water forms a co-ordinate bond to the metal ion metal ions accept the lone pair - Lewis Acids ...
No Slide Title
... aqueous metal ions attract water molecules many have six water molecules surrounding them these are known as hexaaqua ions they are octahedral in shape water acts as a Lewis Base – a lone pair donor water forms a co-ordinate bond to the metal ion metal ions accept the lone pair - Lewis Acids ...
... aqueous metal ions attract water molecules many have six water molecules surrounding them these are known as hexaaqua ions they are octahedral in shape water acts as a Lewis Base – a lone pair donor water forms a co-ordinate bond to the metal ion metal ions accept the lone pair - Lewis Acids ...
Organometallic C-H Bond Activation: An Introduction
... In the sulfuric acid systems, as described above, the acid can act as both the solvent and the oxidant. Common to all strong acid reactions, however, is the fact that strong acids, and even their conjugate bases, are weak ligands (although highly polar); therefore the electrophilicity of the metal i ...
... In the sulfuric acid systems, as described above, the acid can act as both the solvent and the oxidant. Common to all strong acid reactions, however, is the fact that strong acids, and even their conjugate bases, are weak ligands (although highly polar); therefore the electrophilicity of the metal i ...
Hypervalent Iodine Reagents in High Valent Transition Metal
... to being at the forefront of modern catalytic method development. This approach has enabled transformations complimentary to those possible via traditional manifolds, most prominently carbon-heteroatom bond formation. Key to the advancement of this chemistry has been the identification of oxidants t ...
... to being at the forefront of modern catalytic method development. This approach has enabled transformations complimentary to those possible via traditional manifolds, most prominently carbon-heteroatom bond formation. Key to the advancement of this chemistry has been the identification of oxidants t ...
Ryoji Noyori - Nobel Lecture
... C(1)–C(1') pivot and C(2 or 2')–P bonds without seriously increasing torsional strain, while the resulting seven-membered chelate rings containing only sp2 carbon atoms are in turn skeletally unambiguous. The chirality of BINAP is transmitted to other metal coordination sites through the chelate str ...
... C(1)–C(1') pivot and C(2 or 2')–P bonds without seriously increasing torsional strain, while the resulting seven-membered chelate rings containing only sp2 carbon atoms are in turn skeletally unambiguous. The chirality of BINAP is transmitted to other metal coordination sites through the chelate str ...
FACTORS AFFECTING THE SORPTION–DESORPTION OF TRACE
... cation-and anion-exchange capacities, and in the binding energies of their sorption sites (Jackson, 1998; Sparks, 2003; Violante et al., 2005a). In fact, even a single mineral (e.g., a noncrystalline Al hydroxide) has different types of sorption sites, spanning a range of binding energies. The impor ...
... cation-and anion-exchange capacities, and in the binding energies of their sorption sites (Jackson, 1998; Sparks, 2003; Violante et al., 2005a). In fact, even a single mineral (e.g., a noncrystalline Al hydroxide) has different types of sorption sites, spanning a range of binding energies. The impor ...
Living ring-opening metathesis polymerization
... metals, Ziegler and Natta found a series of titanium salts that could polymerize ethylene and propylene to high polymer; work that served as the basis for much of the present polyolefin industry and culminated in the 1963 Nobel Prize in Chemistry [18]. The olefin metathesis reaction was discovered ser ...
... metals, Ziegler and Natta found a series of titanium salts that could polymerize ethylene and propylene to high polymer; work that served as the basis for much of the present polyolefin industry and culminated in the 1963 Nobel Prize in Chemistry [18]. The olefin metathesis reaction was discovered ser ...
+ H 2 O(l) - Knockhardy
... aqueous metal ions attract water molecules many have six water molecules surrounding them these are known as hexaaqua ions they are octahedral in shape water acts as a Lewis Base – a lone pair donor water forms a co-ordinate bond to the metal ion metal ions accept the lone pair - Lewis Acids ...
... aqueous metal ions attract water molecules many have six water molecules surrounding them these are known as hexaaqua ions they are octahedral in shape water acts as a Lewis Base – a lone pair donor water forms a co-ordinate bond to the metal ion metal ions accept the lone pair - Lewis Acids ...
Manganese catalysts in homogeneous oxidation reactions Brinksma
... In Nature, many enzymes are present which are capable of catalysing oxidation reactions.1 In a number of these reactions manganese or iron containing enzymes are involved. These enzymes are frequently studied by using model complexes which provide information on the nature and reactivity of the acti ...
... In Nature, many enzymes are present which are capable of catalysing oxidation reactions.1 In a number of these reactions manganese or iron containing enzymes are involved. These enzymes are frequently studied by using model complexes which provide information on the nature and reactivity of the acti ...
Polyhedral Oligomeric Silsesquioxane
... value is much lower than the expected 1.63 for a hard-sphere, closed-packing mode. Considering the size of alkyl POSS, we concluded that there are small cavities in an alkyl POSS crystal that are occupied by solvent molecules. This result can be attributed to the fact that POSS molecules tend to abs ...
... value is much lower than the expected 1.63 for a hard-sphere, closed-packing mode. Considering the size of alkyl POSS, we concluded that there are small cavities in an alkyl POSS crystal that are occupied by solvent molecules. This result can be attributed to the fact that POSS molecules tend to abs ...
Theoretical studies of nitrilotriacetic acid and nitrilotripropionic acid geometries for
... tasks. Over the years computers continued getting faster and more advanced. We are now at a point in time where one can easily obtain a computer whose computational speed was unimaginable a few years ago. With this increase in speed it is now possible to perform computational calculations on molecul ...
... tasks. Over the years computers continued getting faster and more advanced. We are now at a point in time where one can easily obtain a computer whose computational speed was unimaginable a few years ago. With this increase in speed it is now possible to perform computational calculations on molecul ...
Burdette, S.C., Walkup, G.K., Spingler, B., Tsien, R.Y. and Lippard, S.J.
... 8-aminoquinoline. Recent investigations into the aqueous binding properties of these compounds have clarified many discrepancies found in the literature on these probes, promoting the accuracy of results for future application of these sensors.5,29 Although quinoline-based probes are useful, these s ...
... 8-aminoquinoline. Recent investigations into the aqueous binding properties of these compounds have clarified many discrepancies found in the literature on these probes, promoting the accuracy of results for future application of these sensors.5,29 Although quinoline-based probes are useful, these s ...
pdf-Dokument - Universität Bonn
... Nitrido-iron and oxo-iron complexes are important species in manifold biological and industrial processes. While nitrido-iron complexes are intermediates in biological and industrial nitrogen fixation, oxo-iron complexes are known to be active species in biological oxidation reactions. Therefore, in ...
... Nitrido-iron and oxo-iron complexes are important species in manifold biological and industrial processes. While nitrido-iron complexes are intermediates in biological and industrial nitrogen fixation, oxo-iron complexes are known to be active species in biological oxidation reactions. Therefore, in ...
Synthetically Important AlkaliMetal Utility Amides: Lithium, Sodium
... that dream remains some way off, it is noteworthy that, together, these three amides account for a huge proportion of known deprotonation applications. It is of course not only the deprotonation reaction which can be executed with alkalimetal utility amides (see Section 2). For example, lithium– hal ...
... that dream remains some way off, it is noteworthy that, together, these three amides account for a huge proportion of known deprotonation applications. It is of course not only the deprotonation reaction which can be executed with alkalimetal utility amides (see Section 2). For example, lithium– hal ...
Reactions of Transition Metal Complexes with Fullerenes (C60, C70
... via the routes outlined above for each of the transition elements across the periodic table. Characterization of the products of the reactions of fullerenes with transition metal complexes has involved an array of structural probes. Single-crystal X-ray diffraction is particularly valuable in provid ...
... via the routes outlined above for each of the transition elements across the periodic table. Characterization of the products of the reactions of fullerenes with transition metal complexes has involved an array of structural probes. Single-crystal X-ray diffraction is particularly valuable in provid ...
Application of Novel Phosphine Ligands in Palladium
... catalysts have advantages compared to their homogeneous counterparts. However, being less selective, for many applications they are not suitable. The construction of complex molecules possessing various functional groups, for instance, requires mild reaction conditions, selective reagents and theref ...
... catalysts have advantages compared to their homogeneous counterparts. However, being less selective, for many applications they are not suitable. The construction of complex molecules possessing various functional groups, for instance, requires mild reaction conditions, selective reagents and theref ...
Ligand
4-3D-balls.png?width=300)
In coordination chemistry, a ligand (/lɪɡənd/) is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from covalent to ionic. Furthermore, the metal-ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic ""ligand.""Metals and metalloids are bound to ligands in virtually all circumstances, although gaseous ""naked"" metal ions can be generated in high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection is a critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environmental chemistry.Ligands are classified in many ways like : their charge, their size (bulk), the identity of the coordinating atom(s), and the number of electrons donated to the metal (denticity or hapticity). The size of a ligand is indicated by its cone angle.