article
... complexes [3, 4, 5, 6, 7, 8, 9, 10, 11, 12] The strong back-bonding of the trifluorophosphine ligand in metal trifluorophosphine complexes can be related to the the electron withdrawing properties of its three highly electronegative fluorine atoms [13, 14, 15, 16, 17, 18, 19, 20, 21, 22]. Thus PF3 l ...
... complexes [3, 4, 5, 6, 7, 8, 9, 10, 11, 12] The strong back-bonding of the trifluorophosphine ligand in metal trifluorophosphine complexes can be related to the the electron withdrawing properties of its three highly electronegative fluorine atoms [13, 14, 15, 16, 17, 18, 19, 20, 21, 22]. Thus PF3 l ...
Hydrides: Solid State Transition Metal Complexes
... reaction, such as sintering powder mixtures of the elements, binary alloys, and/or binary metal hydrides at relatively high pressures (up to 160 bar) and moderate temperatures (<1000 K). Synthesis from binary metal compounds is rarely possible because such compounds either do not exist, such as in t ...
... reaction, such as sintering powder mixtures of the elements, binary alloys, and/or binary metal hydrides at relatively high pressures (up to 160 bar) and moderate temperatures (<1000 K). Synthesis from binary metal compounds is rarely possible because such compounds either do not exist, such as in t ...
Ruthenium Olefin Metathesis Catalysts: Tuning of the Ligand Environment Ruthenium olefine
... aiming at the development of new catalysts, one should not only focus on catalyst activity or stability, as highly sophisticated and well-defined initiators are sometimes too expensive for applications on an industrial scale. Other important aspects deserving consideration are the cost to make the c ...
... aiming at the development of new catalysts, one should not only focus on catalyst activity or stability, as highly sophisticated and well-defined initiators are sometimes too expensive for applications on an industrial scale. Other important aspects deserving consideration are the cost to make the c ...
IOSR Journal of Applied Chemistry (IOSR-JAC)
... standard index was calculated as the ratio of the diameter of the inhibitory zone at 1000µg of the complex to that of the standard multiplied by a factor of 10. A comparative ratio of the activities at 1000µg with respect to standard antibiotic ampicillin in terms of the diameter of the inhibitory z ...
... standard index was calculated as the ratio of the diameter of the inhibitory zone at 1000µg of the complex to that of the standard multiplied by a factor of 10. A comparative ratio of the activities at 1000µg with respect to standard antibiotic ampicillin in terms of the diameter of the inhibitory z ...
Fragmentation and charge transfer in gas
... sharply at n 4±6. The actual value depends on the particular metal, the examples of extraordinarily pronounced (over 99% abundance) magic numbers of ®ve (Cu) and six (Ni) are presented in Fig. 1. Hence, generation of ions with only one or two acetonitrile ligands requires enhancing the desolvation ...
... sharply at n 4±6. The actual value depends on the particular metal, the examples of extraordinarily pronounced (over 99% abundance) magic numbers of ®ve (Cu) and six (Ni) are presented in Fig. 1. Hence, generation of ions with only one or two acetonitrile ligands requires enhancing the desolvation ...
Cadmiumcomplexes bearing N^E^O (E = S, Se
... On the other hand, the amino side-arm does not bind to the metal atom, with the dCd1–N2 distance (6.105(3) Å) far exceeding the sum of the van der Waals radii for these elements. The dCd1–N1 bond distance (2.108(3) Å) is typical of terminal [Cd]–N(SiMe3)2 amides.3-6 The geometry about the cadmium a ...
... On the other hand, the amino side-arm does not bind to the metal atom, with the dCd1–N2 distance (6.105(3) Å) far exceeding the sum of the van der Waals radii for these elements. The dCd1–N1 bond distance (2.108(3) Å) is typical of terminal [Cd]–N(SiMe3)2 amides.3-6 The geometry about the cadmium a ...
Stoichiometry - Mr Field`s Chemistry Class
... Group 1: Explain the physical states (under standard conditions) and electrical conductivity (in the molten state) of the chlorides and oxides of the elements in period 3 in terms of their bonding and structure. Group 2: Describe the reactions of chlorine and the chlorides referred to above with wat ...
... Group 1: Explain the physical states (under standard conditions) and electrical conductivity (in the molten state) of the chlorides and oxides of the elements in period 3 in terms of their bonding and structure. Group 2: Describe the reactions of chlorine and the chlorides referred to above with wat ...
Chemical Speciation of Sulfur and Metals in Biogas
... microbial pathways initiated by hydrolysis of organic composites i.e. carbohydrates, proteins, and lipids into soluble mono- and oligomers such as sugars, amino acids, and fatty acids (Ahring 2003). The hydrolytic products are subsequently degraded into intermediate fermentation products such as ace ...
... microbial pathways initiated by hydrolysis of organic composites i.e. carbohydrates, proteins, and lipids into soluble mono- and oligomers such as sugars, amino acids, and fatty acids (Ahring 2003). The hydrolytic products are subsequently degraded into intermediate fermentation products such as ace ...
here - University of Alberta
... in solution at ambient temperature.67 As is also typical of binuclear species bridged by only one bidentate ligand, the dicarbene framework in 7 is twisted in such a way to allow the metal coordination planes to avoid each other. As a result the Rh(1)- - -Rh(2) separation is quite large, at 7.137(1) ...
... in solution at ambient temperature.67 As is also typical of binuclear species bridged by only one bidentate ligand, the dicarbene framework in 7 is twisted in such a way to allow the metal coordination planes to avoid each other. As a result the Rh(1)- - -Rh(2) separation is quite large, at 7.137(1) ...
Lanthanides and actinides. Annual survey of their organometallic
... This review has been restricted to compounds of the lanthanides and actinides containing M-C bonds as defined by section 29 of Chemical Abstracts. The prelanthanides SC, Y, and La have been included because of their similar size and charge to the lanthanides. Abstracts of papers presented at confere ...
... This review has been restricted to compounds of the lanthanides and actinides containing M-C bonds as defined by section 29 of Chemical Abstracts. The prelanthanides SC, Y, and La have been included because of their similar size and charge to the lanthanides. Abstracts of papers presented at confere ...
Activation of Carbon-Fluorine Bonds by Metal
... A).29 As such, fluorine has the unusual ability to completely substitute for hydrogen in organic hydrocarbons without causing any gross geometrical distortions. The replacement of hydrogen with fluorine results in a marked change in the physical and chemicalproperties of hydrocarbons versus fluoroca ...
... A).29 As such, fluorine has the unusual ability to completely substitute for hydrogen in organic hydrocarbons without causing any gross geometrical distortions. The replacement of hydrogen with fluorine results in a marked change in the physical and chemicalproperties of hydrocarbons versus fluoroca ...
Pentadentate thioether-oxime macrocyclic and quasi-macrocyclic complexes of copper(II) and nickel(II)
... An ORTEP projection is shown in Fig. 2, while selected bond distances and angles are given in Table 2. The X-ray structure reveals an almost perfect square pyramidal N2S3 coordination environment (t=0.06) [22]. The Cu(II) in each quasi-macrocyclic unit is coordinated by two cis-thioether sulfur and ...
... An ORTEP projection is shown in Fig. 2, while selected bond distances and angles are given in Table 2. The X-ray structure reveals an almost perfect square pyramidal N2S3 coordination environment (t=0.06) [22]. The Cu(II) in each quasi-macrocyclic unit is coordinated by two cis-thioether sulfur and ...
reactivity of heterocyclic compounds
... Heterocycles can act bound to metals through their lone pairs (Bronsted basicity) forming coordination compounds ...
... Heterocycles can act bound to metals through their lone pairs (Bronsted basicity) forming coordination compounds ...
Valence, Oxidation Number, and Formal Charge: Three Related but
... The coordination number is simply defined as the number of atoms attached to the atom of interest in a molecule (15). For molecules of the type AHn the coordination number of A, n, is equivalent to its valence. However, the equivalence between valence and coordination number breaks down when a multi ...
... The coordination number is simply defined as the number of atoms attached to the atom of interest in a molecule (15). For molecules of the type AHn the coordination number of A, n, is equivalent to its valence. However, the equivalence between valence and coordination number breaks down when a multi ...
2 - Royal Society of Chemistry
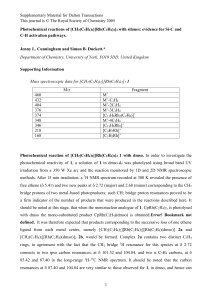
... Supplementary Material for Dalton Transactions This journal is © The Royal Society of Chemistry 2005 signals 1.42 and -0.18, and 2.89 and 2.53. This supports the idea that both the arene and alkene rotate. However, the signals at 1.42 and 2.89 had a weak doublet splitting, in contrast to the ...
... Supplementary Material for Dalton Transactions This journal is © The Royal Society of Chemistry 2005 signals 1.42 and -0.18, and 2.89 and 2.53. This supports the idea that both the arene and alkene rotate. However, the signals at 1.42 and 2.89 had a weak doublet splitting, in contrast to the ...
The Use of XAFS to Distinguish between Inner- and Outer
... mineral having an ideal half cell chemical formula M 0.33 , H 2O Si 4(Al 1.67,(Fe 21, Mg) 0.33)O 10(OH) 2 (2), where M is a monovalent interlayer cation (2). Figure 1 shows the molecular structure along the (010) plane of montmorillonite. Substitution of Fe 21 and Mg 21 atoms for Al 31 in the octahe ...
... mineral having an ideal half cell chemical formula M 0.33 , H 2O Si 4(Al 1.67,(Fe 21, Mg) 0.33)O 10(OH) 2 (2), where M is a monovalent interlayer cation (2). Figure 1 shows the molecular structure along the (010) plane of montmorillonite. Substitution of Fe 21 and Mg 21 atoms for Al 31 in the octahe ...
University of Groningen Reactivity of rare earth metal
... The catalytic dimerization of terminal alkynes is a straightforward and atom-economical method to synthesize conjugated enyne motifs, which are important building blocks for organic synthesis and key units found in a variety of biologically active compounds and synthetic conjugated polymers for opto ...
... The catalytic dimerization of terminal alkynes is a straightforward and atom-economical method to synthesize conjugated enyne motifs, which are important building blocks for organic synthesis and key units found in a variety of biologically active compounds and synthetic conjugated polymers for opto ...
Synthesis of DiamidoPyrrolyl Molybdenum Complexes Relevant to Reduction DOI: 10.1021/ic100856n
... The chemical shift of the dimethylamido protons in the proton NMR spectrum of [(C6F5N)2Pyr]Mo(NMe2) is temperature dependent, a phenomenon that is analogous to what is found for the triamidoamine complexes, [TMSN3N]Mo(NMe2) ([TMSN3N]3- = [(Me3SiNCH2CH2)3N]3-)19 and [C6F5N3N]Mo(NMe2),20 and which is ...
... The chemical shift of the dimethylamido protons in the proton NMR spectrum of [(C6F5N)2Pyr]Mo(NMe2) is temperature dependent, a phenomenon that is analogous to what is found for the triamidoamine complexes, [TMSN3N]Mo(NMe2) ([TMSN3N]3- = [(Me3SiNCH2CH2)3N]3-)19 and [C6F5N3N]Mo(NMe2),20 and which is ...
Nomenclature of Inorganic Chemistry (IUPAC Recommendations
... IR-10). Overall, the emphasis on additive nomenclature (generalized from the classical nomenclature of coordination compounds) which was already apparent in Red Book I is reinforced here. Examples are even included of organic compounds, from the borderline between inorganic and organic chemistry, wh ...
... IR-10). Overall, the emphasis on additive nomenclature (generalized from the classical nomenclature of coordination compounds) which was already apparent in Red Book I is reinforced here. Examples are even included of organic compounds, from the borderline between inorganic and organic chemistry, wh ...
Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005
... IR-10). Overall, the emphasis on additive nomenclature (generalized from the classical nomenclature of coordination compounds) which was already apparent in Red Book I is reinforced here. Examples are even included of organic compounds, from the borderline between inorganic and organic chemistry, wh ...
... IR-10). Overall, the emphasis on additive nomenclature (generalized from the classical nomenclature of coordination compounds) which was already apparent in Red Book I is reinforced here. Examples are even included of organic compounds, from the borderline between inorganic and organic chemistry, wh ...
Chapter 16 Solubility Equilibrium
... • For example, AgCl has a much lower Ksp than PbCl2 • If Ag+ and Pb2+ are present in the same solution, the Ag+ ion can be selectively precipitated as AgCl, leaving Pb2+ in solution. ...
... • For example, AgCl has a much lower Ksp than PbCl2 • If Ag+ and Pb2+ are present in the same solution, the Ag+ ion can be selectively precipitated as AgCl, leaving Pb2+ in solution. ...
THESE DOCTORAT DE L`UNIVERSITE DE TOULOUSE ET
... The chemistry of molybdenum and tungsten cyclopentadienyl complexes in higher oxidation states with oxo, imido and sulfide ligands has increased in significance. Interest in Cp* oxo molybdenum and tungsten complexes is particularly motivated by their potential in oxidation catalysis. Most advances i ...
... The chemistry of molybdenum and tungsten cyclopentadienyl complexes in higher oxidation states with oxo, imido and sulfide ligands has increased in significance. Interest in Cp* oxo molybdenum and tungsten complexes is particularly motivated by their potential in oxidation catalysis. Most advances i ...
Ligand
4-3D-balls.png?width=300)
In coordination chemistry, a ligand (/lɪɡənd/) is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from covalent to ionic. Furthermore, the metal-ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic ""ligand.""Metals and metalloids are bound to ligands in virtually all circumstances, although gaseous ""naked"" metal ions can be generated in high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection is a critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environmental chemistry.Ligands are classified in many ways like : their charge, their size (bulk), the identity of the coordinating atom(s), and the number of electrons donated to the metal (denticity or hapticity). The size of a ligand is indicated by its cone angle.