2 - A-Level Chemistry
... Deduce the formula of the linear complex formed when an excess of concentrated hydrochloric acid is added to silver chloride. ...
... Deduce the formula of the linear complex formed when an excess of concentrated hydrochloric acid is added to silver chloride. ...
CHEMISTRY notes
... The reaction can “shift” left or right under certain conditions EXCESS REACTANT shifts right (excess product, left) REMOVING REACTANT shifts left (removing product, right) ADDING/REMOVING ENERGY can shift it as well http://www.youtube.com/watch?v=a_4LUaaL6FU&feature=fvsr (blue bottle) http://www.you ...
... The reaction can “shift” left or right under certain conditions EXCESS REACTANT shifts right (excess product, left) REMOVING REACTANT shifts left (removing product, right) ADDING/REMOVING ENERGY can shift it as well http://www.youtube.com/watch?v=a_4LUaaL6FU&feature=fvsr (blue bottle) http://www.you ...
Oxidation Number Rules
... o -2 in all other compounds...most common 4. Hydrogen (H) has two possible oxidation numbers: o +1 when bonded to a nonmetal o -1 when bonded to a metal 5. The oxidation number of fluorine (F) is always -1. All other halogens have a -1 oxidation number in compounds, except when with oxygen or other ...
... o -2 in all other compounds...most common 4. Hydrogen (H) has two possible oxidation numbers: o +1 when bonded to a nonmetal o -1 when bonded to a metal 5. The oxidation number of fluorine (F) is always -1. All other halogens have a -1 oxidation number in compounds, except when with oxygen or other ...
Chapter 2 Practice Questions
... E) tend to gain electrons in chemical reactions 14. You are given a compound with the formula MCl2, in which M is a metal. You are told that the metal ion has 26 electrons. What is the identity of the metal? A) Fe B) Al C) Zn D) Co E) Ni ...
... E) tend to gain electrons in chemical reactions 14. You are given a compound with the formula MCl2, in which M is a metal. You are told that the metal ion has 26 electrons. What is the identity of the metal? A) Fe B) Al C) Zn D) Co E) Ni ...
CHEM_1305_Practice_Exam_2
... 19) Which of the statements below best describes the following reaction? Na2CO3(s) Na2O(s) + CO2(g) A) Solid sodium carbonate decomposes to give solid sodium oxide and carbon dioxide gas. B) Sodium carbonate decomposes to form sodium oxide and carbon dioxide. C) Sodium oxide combines with carbon d ...
... 19) Which of the statements below best describes the following reaction? Na2CO3(s) Na2O(s) + CO2(g) A) Solid sodium carbonate decomposes to give solid sodium oxide and carbon dioxide gas. B) Sodium carbonate decomposes to form sodium oxide and carbon dioxide. C) Sodium oxide combines with carbon d ...
Model Description Sheet
... Primary Citation: Abe, C., Muto, Y., Inoue, M., Kigawa, T., Terada, T., Shirouzu, M., Yokoyama, S. Solution structure of the RNA binding domain in Heterogeneous nuclear ribonucleoprotein M. To be published. Format: Alpha carbon backbone RP: Zcorp with plaster Description: Nitrogen and oxygen are two ...
... Primary Citation: Abe, C., Muto, Y., Inoue, M., Kigawa, T., Terada, T., Shirouzu, M., Yokoyama, S. Solution structure of the RNA binding domain in Heterogeneous nuclear ribonucleoprotein M. To be published. Format: Alpha carbon backbone RP: Zcorp with plaster Description: Nitrogen and oxygen are two ...
E16 THE COPPER(II) SULFATE/AMMONIA COMPLEX A complex
... include the hydroxide ion (OH–), water molecule (H2O), ammonia molecule (NH3), cyanide ion (CN–) and halide ions (F–, Cl–, Br– and I–). ...
... include the hydroxide ion (OH–), water molecule (H2O), ammonia molecule (NH3), cyanide ion (CN–) and halide ions (F–, Cl–, Br– and I–). ...
Document
... include the hydroxide ion (OH–), water molecule (H2O), ammonia molecule (NH3), cyanide ion (CN–) and halide ions (F–, Cl–, Br– and I–). ...
... include the hydroxide ion (OH–), water molecule (H2O), ammonia molecule (NH3), cyanide ion (CN–) and halide ions (F–, Cl–, Br– and I–). ...
Worksheet 1 - Ch. 2, 3 - Iowa State University
... b. Peptide bonds form between __________ and ___________ functional groups. 3. This is an image of an aquaporin protein; it lets water into the cell through a tunnel. a. What structure does it have? b. Determine whether polar and nonpolar amino acids would be located on the inside or outer layer of ...
... b. Peptide bonds form between __________ and ___________ functional groups. 3. This is an image of an aquaporin protein; it lets water into the cell through a tunnel. a. What structure does it have? b. Determine whether polar and nonpolar amino acids would be located on the inside or outer layer of ...
Self-assessment quiz for young scientist interested in autumn school
... 7. The sequence in question 6. has been taken from a real protein for which an experimental Xray structure exists. From which protein? 8. You have the amino acid sequences of two large proteins A and B. Someone claims: A and B are totally different except for one domain, for which homologous domains ...
... 7. The sequence in question 6. has been taken from a real protein for which an experimental Xray structure exists. From which protein? 8. You have the amino acid sequences of two large proteins A and B. Someone claims: A and B are totally different except for one domain, for which homologous domains ...
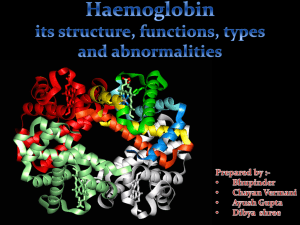
Haemoglobin (Roll no. 22
... molecule is an assembly of four globular protein subunits. Each subunit is composed of a protein chain tightly associated with a non-protein heme group. Each protein chain arranges into a set of alpha-helix , structural segments connected together in a globin fold arrangement. This folding pattern c ...
... molecule is an assembly of four globular protein subunits. Each subunit is composed of a protein chain tightly associated with a non-protein heme group. Each protein chain arranges into a set of alpha-helix , structural segments connected together in a globin fold arrangement. This folding pattern c ...
2.8-Reaction Rate
... types of chemical reactions: 1) Corrosion – the deterioration of metals. It always occurs in the presence of oxygen. Example: Fe + O2 Fe2O3 Metal + oxygen (corrosion) ...
... types of chemical reactions: 1) Corrosion – the deterioration of metals. It always occurs in the presence of oxygen. Example: Fe + O2 Fe2O3 Metal + oxygen (corrosion) ...
Lecture 29
... Val E11 out of the oxygen’s path to the Fe on the other subunit. This process increases the affinity of the heme toward oxygen. The a1-b2 contacts have two stable positions . These contacts, which are joined by different but equivalent sets of hydrogen-bonds that act as a binary switch between the T ...
... Val E11 out of the oxygen’s path to the Fe on the other subunit. This process increases the affinity of the heme toward oxygen. The a1-b2 contacts have two stable positions . These contacts, which are joined by different but equivalent sets of hydrogen-bonds that act as a binary switch between the T ...
Final Review: L17-25
... HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l) The total ionic equation is: H+(aq) + Cl-(aq) + Na+(aq) + OH-(aq) → Na+(aq) + Cl-(aq) + H2O(l) ...
... HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l) The total ionic equation is: H+(aq) + Cl-(aq) + Na+(aq) + OH-(aq) → Na+(aq) + Cl-(aq) + H2O(l) ...
Organic Chem Biology
... cell membranes, as antibodies to fight disease, and as enzymes to catalyze metabolic reactions and cell processes. ...
... cell membranes, as antibodies to fight disease, and as enzymes to catalyze metabolic reactions and cell processes. ...
tetrahedral site
... tetrahedral site: Fe ion is surrounded by four oxygens octahedral site: Fe ion is surrounded by six oxygens The tetrahedral and octahedral sites form the two magnetic sublattices, A and B respectively. The spins on the A sublattice are antiparallel to those on the B sublattice. The two crystal sites ...
... tetrahedral site: Fe ion is surrounded by four oxygens octahedral site: Fe ion is surrounded by six oxygens The tetrahedral and octahedral sites form the two magnetic sublattices, A and B respectively. The spins on the A sublattice are antiparallel to those on the B sublattice. The two crystal sites ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.