* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File
Hydroformylation wikipedia , lookup
Metal carbonyl wikipedia , lookup
Spin crossover wikipedia , lookup
Cluster chemistry wikipedia , lookup
Bond valence method wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
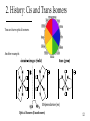
Coordination Chemistry: History and Introduction to Structure 1 1. Coordination Compounds Involves: Metal atoms or ions One or more ligands (atoms, ions, or molecules) that donate e- to the metal Chemistry of the metal d-orbitals Consist of the formation of coordinate covalent (dative) bonds: M + L M-L Lewis Acid-Base Adduct Metal is the Lewis Acid Ligand is the Lewis Base 2 2. History Complimentary Color Wheel A. A tale as old as time (Prehistoric) Absorb B. Formally introduced by Alfred Werner (late 19th Century) Introduced new bonding concepts The famous Werner Cobalt Compounds Compound A B C D Elemental Formula CoN6H18Cl3 CoN5H15Cl3 CoN4H12Cl3 CoN3H9Cl3 See Color Yellow Red Green or Purple 3 2. History: Werner Compounds Compound A B C D Elemental Formula CoN6H18Cl3 CoN5H15Cl3 CoN4H12Cl3 CoN3H9Cl3 Color Yellow Red Green or Purple Early bonding theories allowed only three atoms to be attached to cobalt because of its valence of 3 (Co3+) for charge balance. Jørgensen proposed that for the above compounds • N could form chains because of its valence of 5 • Chloride (Cl-) could be bound to N or to Co3+ Werner proposed something very radical for the time • As many as 6 N (as NH3) could bond directly to Co3+ • Cl- could bond to Co3+ or associate loosely; two kinds of Cl- 4 2. History: The Werner Titrations Complex (aq) + AgNO3 (aq) Compound If Cl- is N bound, would dissociate in water. Recall: There are 2 Isomers Note: 0 Cl-? Jørgensen AgCl equivs A 3 B 2 C 1 D 0 n AgCl (s) + Complexn+ Werner 5 2. History: Werner’s Theory [Co(NH3)6]Cl3 A. Primary Bonding The positive charge of the metal ion is balanced by negative ions. Does not have to involve direct bonding to the metal ion. Primary Sphere • Today when direct bonding is not involved, we refer to it as the secondary coordination sphere. Secondary Sphere B. Secondary Bonding Ligands (molecules or ions) directly attached to the metal ion. This interaction constitutes the coordination sphere; the complex ion. • Today we refer to it as the primary coordination sphere. Defines the coordination number. Defines a specific geometry; “directed in space”. Remember these are older theories 6 2. History: Determining the coordination mode Which coordination mode would give 2 isomers for complex C? a b c d 7 8 9 10 trans cis Yes! 11 2. History: Cis and Trans Isomers You can have optical isomers. Another example. Ethylenediamine (en) Optical Isomers (Enantiomers) 12













![Coordination Compounds [Compatibility Mode]](http://s1.studyres.com/store/data/000678035_1-c20c75fd4abb97d3ba4a0b0fce26e10b-150x150.png)








