4.IonicCompounds - Gleneaglesunit1and2chemistry2012
... • Ionic bonds formed when metal atoms combined with non-metal atoms • Metallic bonds formed when metal atoms combined with metal atoms. • Covalent bonds formed when non-metal atoms combined with non-metal atoms. ...
... • Ionic bonds formed when metal atoms combined with non-metal atoms • Metallic bonds formed when metal atoms combined with metal atoms. • Covalent bonds formed when non-metal atoms combined with non-metal atoms. ...
Midterm for Bio98B A1 (1) Enzymes accelerate reactions by
... Midterm for Bio98B A1 (1) Enzymes accelerate reactions by decreasing the free energy input needed to achieve ...
... Midterm for Bio98B A1 (1) Enzymes accelerate reactions by decreasing the free energy input needed to achieve ...
Biochemistry 6/e
... catalytic activity - Carbonic anhydrase contains Zinc ion; first known zinc-containing enzyme. - Zinc ion is necessary for catalytic activity. - At least 7 carbonic anhydrase in human. - Carbonic anhydrase Ⅱ is the most extensively studied. - Three coordination sites are occupied by the imidazole ri ...
... catalytic activity - Carbonic anhydrase contains Zinc ion; first known zinc-containing enzyme. - Zinc ion is necessary for catalytic activity. - At least 7 carbonic anhydrase in human. - Carbonic anhydrase Ⅱ is the most extensively studied. - Three coordination sites are occupied by the imidazole ri ...
ATOMS, MOLECULES, AND IONS
... kg. Later experiments by Rutherford determined that at the center of an atom is a positively charged, compact, heavy nucleus. The charge on the atomic nucleus is +Ze (Z is the atomic number of the atom). The fundamental unit of positive charge in the nucleus is the proton. ♦ Chemical identity of an ...
... kg. Later experiments by Rutherford determined that at the center of an atom is a positively charged, compact, heavy nucleus. The charge on the atomic nucleus is +Ze (Z is the atomic number of the atom). The fundamental unit of positive charge in the nucleus is the proton. ♦ Chemical identity of an ...
Samples Ch 10 to 12.tst
... There will be 7 turns of the beta oxidation and formation of 8 acetyl CoA molecules: therefore the 8 acetyl CoA will yield 80 ATP, and then 17.5 ATP from the NADH of the cycle and 10.5 ATP from the FADH2 . The final total would ...
... There will be 7 turns of the beta oxidation and formation of 8 acetyl CoA molecules: therefore the 8 acetyl CoA will yield 80 ATP, and then 17.5 ATP from the NADH of the cycle and 10.5 ATP from the FADH2 . The final total would ...
AP Chemistry Summer Assignment - 2015
... EX. Ca(OH)2(s) → CaO(s) + H2O(g) 3. Metallic chlorates, when heated, decompose into metallic chlorides and oxygen gas. EX. 2KClO3(s) → 2KCl(s) + 3O2(g) 4. Some acids, when heated, decompose into nonmetallic oxides and water. EX. H2SO4 → H2O(l) + SO3(g) 5. Some oxides, when heated, decompose to the e ...
... EX. Ca(OH)2(s) → CaO(s) + H2O(g) 3. Metallic chlorates, when heated, decompose into metallic chlorides and oxygen gas. EX. 2KClO3(s) → 2KCl(s) + 3O2(g) 4. Some acids, when heated, decompose into nonmetallic oxides and water. EX. H2SO4 → H2O(l) + SO3(g) 5. Some oxides, when heated, decompose to the e ...
Periodicity (AHL)
... Why not 4 or 8? Six is the maximum number of water molecules it is possible to fit around an iron ion (and most other metal ions). By making the maximum number of bonds, it releases most energy and so becomes most energetically stable. ...
... Why not 4 or 8? Six is the maximum number of water molecules it is possible to fit around an iron ion (and most other metal ions). By making the maximum number of bonds, it releases most energy and so becomes most energetically stable. ...
Ch 2 BS
... atom Red gives up an electron to another atom to form a bond, the Red atom will now have a positive charge. If Yellow atom takes the extra electron and forms a bond, the Yellow atom will now have a negative charge. ...
... atom Red gives up an electron to another atom to form a bond, the Red atom will now have a positive charge. If Yellow atom takes the extra electron and forms a bond, the Yellow atom will now have a negative charge. ...
Naming Ionic Compounds
... ** this is just like you learned for molecular compounds except you are not worried about the numbers of an element examples: NaCl – sodium chloride CaCl2 – calcium chloride Mg3N2 – magnesium nitride PbO – lead oxide ...
... ** this is just like you learned for molecular compounds except you are not worried about the numbers of an element examples: NaCl – sodium chloride CaCl2 – calcium chloride Mg3N2 – magnesium nitride PbO – lead oxide ...
Classification and Nomenclature of Enzymes
... where “a” is the class, “b” is the subclass, “c” is the sub‐subclass, and “d” is the sub‐sub‐subclass. The “b” and “c” digits describe the reaction, while the “d” digit is used to distinguish between different enzymes of the same function based on the actual substrate in the reaction. • Exampl ...
... where “a” is the class, “b” is the subclass, “c” is the sub‐subclass, and “d” is the sub‐sub‐subclass. The “b” and “c” digits describe the reaction, while the “d” digit is used to distinguish between different enzymes of the same function based on the actual substrate in the reaction. • Exampl ...
Isotope - Northern Highlands
... 5. Describe 2 biological uses for radioisotopes. 6. What are some potential risk factors of uncontrolled exposure to radioisotopes? CHEMICAL COMPOUNDS 1. Define compound. 2. In what ways do compounds differ from their component elements? CHEMICAL BONDS 1. Define ion and ionic bonds. Give several exa ...
... 5. Describe 2 biological uses for radioisotopes. 6. What are some potential risk factors of uncontrolled exposure to radioisotopes? CHEMICAL COMPOUNDS 1. Define compound. 2. In what ways do compounds differ from their component elements? CHEMICAL BONDS 1. Define ion and ionic bonds. Give several exa ...
Toward detection of DNA-bound proteins using solid-state
... Movie showing a MD simulation of the nanopore-induced rupture of a protein-DNA complex. First, a cross section of the nanopore is shown. Next, ions moving in the electric field transverse to the membrane are shown. Although ions and water are not shown during the whole video, they were always presen ...
... Movie showing a MD simulation of the nanopore-induced rupture of a protein-DNA complex. First, a cross section of the nanopore is shown. Next, ions moving in the electric field transverse to the membrane are shown. Although ions and water are not shown during the whole video, they were always presen ...
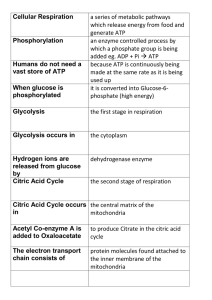
File
... added eg. ADP + Pi ATP because ATP is continuously being made at the same rate as it is being used up it is converted into Glucose-6phosphate (high energy) ...
... added eg. ADP + Pi ATP because ATP is continuously being made at the same rate as it is being used up it is converted into Glucose-6phosphate (high energy) ...
Complex Ions
... What is a Complex Ion? A property of transition metals is their ability to form complex ions Complex ion:Central metal ion is surrounded by ligands Ligand: Molecule / ion which donates a pair of electrons forming a coordinate bond (dative bond) Coordinate bond: One of the bonded atoms has provided ...
... What is a Complex Ion? A property of transition metals is their ability to form complex ions Complex ion:Central metal ion is surrounded by ligands Ligand: Molecule / ion which donates a pair of electrons forming a coordinate bond (dative bond) Coordinate bond: One of the bonded atoms has provided ...
Chem 30A Final Exam
... indicate whether the compound involves ionic bonding, covalent bonding, or both. formula? ionic? covalent? both? potassium carbonate ...
... indicate whether the compound involves ionic bonding, covalent bonding, or both. formula? ionic? covalent? both? potassium carbonate ...
Chem 1411 Chapt2
... Types of CompoundsIonic- Consists of metals and non-metals (Or in general cations and anions). NaCl, MgCl2, K2S, Na2SO4 Molecular (covalent)- Consists of non-metals only. HCl, N2O4, C3H6O, C6H12O6 Note- All compounds can be molecules; not all molecules can be compounds. Ions- Are chemical species th ...
... Types of CompoundsIonic- Consists of metals and non-metals (Or in general cations and anions). NaCl, MgCl2, K2S, Na2SO4 Molecular (covalent)- Consists of non-metals only. HCl, N2O4, C3H6O, C6H12O6 Note- All compounds can be molecules; not all molecules can be compounds. Ions- Are chemical species th ...
Intro to Biochemistry Pratt & Cornely Chapter 1
... The reaction above can be coupled to a reaction in which 1,3-BPG is converted to 3PG. Write the overall equation, including the change in free energy for the converstion of GAP to 3PG. 1,3BPG + ADP 3PG + ATP DG = -18.8 kJ/mol ...
... The reaction above can be coupled to a reaction in which 1,3-BPG is converted to 3PG. Write the overall equation, including the change in free energy for the converstion of GAP to 3PG. 1,3BPG + ADP 3PG + ATP DG = -18.8 kJ/mol ...
File
... 1. Polymers are chains of repeating units (poly means “many”) 2. The units they’re made up of are called monomers (mono means “one”) 3. Take a brick wall, for example: each individual brick would be a monomer. The wall would be the polymer. ...
... 1. Polymers are chains of repeating units (poly means “many”) 2. The units they’re made up of are called monomers (mono means “one”) 3. Take a brick wall, for example: each individual brick would be a monomer. The wall would be the polymer. ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.