Alkene reaction study guide
... Tips for Multistep Synthesis Problems: o Work Backwards – analyze the product and look for something “special” (alcohols, cyclopropane). o Think which reactions could have yielded the product given. o Look for differences between the original and the final compounds (for example, if there is a chlor ...
... Tips for Multistep Synthesis Problems: o Work Backwards – analyze the product and look for something “special” (alcohols, cyclopropane). o Think which reactions could have yielded the product given. o Look for differences between the original and the final compounds (for example, if there is a chlor ...
Chemistry 160 Th 13 Mar 2008 In-Class Worksheet A. Each of the
... Ethene contains a double bond, and ethane has a single bond between the two carbons. In order to create a double bond, the carbons overlap their p orbitals, which are perpendicular to the sigma bond formed between the carbons. The reason that no rotation can occur between the carbons is that rotatio ...
... Ethene contains a double bond, and ethane has a single bond between the two carbons. In order to create a double bond, the carbons overlap their p orbitals, which are perpendicular to the sigma bond formed between the carbons. The reason that no rotation can occur between the carbons is that rotatio ...
Preparation of alkyl halides There are lots of ways to make alkyl
... You use some kind of base in each of these cases (either triethylamine or pyridine) so that you can neutralize the acid that is formed during the reaction. The key feature of these reactions is that you are converting OH into a much better leaving group as well. 2. Preparation o ...
... You use some kind of base in each of these cases (either triethylamine or pyridine) so that you can neutralize the acid that is formed during the reaction. The key feature of these reactions is that you are converting OH into a much better leaving group as well. 2. Preparation o ...
Johnson Group Research
... organic transformations. One area of current interest is the development of methodology for the cleavage and functionalization of carbon-carbon bonds. While carbon-carbon single bonds are inert under a vast majority of standard reaction conditions, certain transition metal complexes promote the acti ...
... organic transformations. One area of current interest is the development of methodology for the cleavage and functionalization of carbon-carbon bonds. While carbon-carbon single bonds are inert under a vast majority of standard reaction conditions, certain transition metal complexes promote the acti ...
Dehydration of 3,3-dimethyl-2-butanol to make alkenes March 1 & 3
... Elimination Reactions Let’s Review: – Elimination reaction: a fundamental organic reaction Two species are eliminated from a substrate Elimination mean’s they are gone, gone, gone – NOT a substitution ...
... Elimination Reactions Let’s Review: – Elimination reaction: a fundamental organic reaction Two species are eliminated from a substrate Elimination mean’s they are gone, gone, gone – NOT a substitution ...
Organic Chemistry
... • A family of hydrocarbons is a homologous series • This means they have the same functional groups • You can have alcohols (ROH), alkanes (RH), Haloalkanes (RX) and MANY OTHERS Roger Frost ...
... • A family of hydrocarbons is a homologous series • This means they have the same functional groups • You can have alcohols (ROH), alkanes (RH), Haloalkanes (RX) and MANY OTHERS Roger Frost ...
Summary from Organic Chemistry Packet:
... • Recognize the terms cis-, trans- isomers – Unsaturated molecules – Orientation around the double bond ...
... • Recognize the terms cis-, trans- isomers – Unsaturated molecules – Orientation around the double bond ...
Document
... trienes, and so forth. • Always choose the longest chain that contains both atoms of the double bond. • In naming cycloalkenes, the double bond is located between C1 and C2, and the “1” is usually omitted in the name. The ring is numbered clockwise or counterclockwise to give the first substituent t ...
... trienes, and so forth. • Always choose the longest chain that contains both atoms of the double bond. • In naming cycloalkenes, the double bond is located between C1 and C2, and the “1” is usually omitted in the name. The ring is numbered clockwise or counterclockwise to give the first substituent t ...
Organic Reactions 2.1- 2.3 - mccormack-sch4u-2013
... 4) OXIDATION & 5) REDUCTION REACTIONS • Change in the number of H or O atoms bonded to C • Always occur together • One reactant is oxidized while the other is reduced • For now, lets focus on reactant only… ...
... 4) OXIDATION & 5) REDUCTION REACTIONS • Change in the number of H or O atoms bonded to C • Always occur together • One reactant is oxidized while the other is reduced • For now, lets focus on reactant only… ...
Test 12
... Amines and Amides Identify an Amino Acid (has amine group and acid group) Organic Reactions Substitution: with alkanes, one atom substitutes for another, often happens with halogens Addition: with alkenes/alkynes, two atoms add on as a double or triple bond is broken Esterification: alcohol + organi ...
... Amines and Amides Identify an Amino Acid (has amine group and acid group) Organic Reactions Substitution: with alkanes, one atom substitutes for another, often happens with halogens Addition: with alkenes/alkynes, two atoms add on as a double or triple bond is broken Esterification: alcohol + organi ...
ch07 by Dr. Dina
... Sodium alkynides can be used as nucleophiles in SN2 reactions New carbon-carbon bonds are the result Only primary alkyl halides can be used or else elimination ...
... Sodium alkynides can be used as nucleophiles in SN2 reactions New carbon-carbon bonds are the result Only primary alkyl halides can be used or else elimination ...
chapter 2: reactions of organic compounds
... 4) OXIDATION & 5) REDUCTION REACTIONS • Change in the number of H or O atoms bonded to C • Always occur together • One reactant is oxidized while the other is reduced • For now, lets focus on reactant only… ...
... 4) OXIDATION & 5) REDUCTION REACTIONS • Change in the number of H or O atoms bonded to C • Always occur together • One reactant is oxidized while the other is reduced • For now, lets focus on reactant only… ...
$doc.title
... http://www.chem.wisc.edu/areas/clc (Resource page) Reactions of Alcohols #8: Reaction of a 1° Alcohol with Hydrogen Halides ...
... http://www.chem.wisc.edu/areas/clc (Resource page) Reactions of Alcohols #8: Reaction of a 1° Alcohol with Hydrogen Halides ...
Organic Notes #5 - RX`ns - Winston Knoll Collegiate
... reacting temperature is relatively high. Carbon dioxide is produced and the oxidation state of carbon is +4. CH4 + 2O2 ➩ CO2 + 2H2O Cracking - when larger hydrocarbons (10-16 carbons) are broken into smaller hydrocarbons. This process is used in the petroleum industry to create a larger number of mo ...
... reacting temperature is relatively high. Carbon dioxide is produced and the oxidation state of carbon is +4. CH4 + 2O2 ➩ CO2 + 2H2O Cracking - when larger hydrocarbons (10-16 carbons) are broken into smaller hydrocarbons. This process is used in the petroleum industry to create a larger number of mo ...
Senior Science topics Programme
... understanding of the reactivity of different chemical species with experimental observations to deduce the most likely sequence of elementary steps and thus the mechanism of a particular reaction. Knowledge of reaction mechanisms facilitates scientists to plan for synthesising new compounds from som ...
... understanding of the reactivity of different chemical species with experimental observations to deduce the most likely sequence of elementary steps and thus the mechanism of a particular reaction. Knowledge of reaction mechanisms facilitates scientists to plan for synthesising new compounds from som ...
Topic 10. Organic chemistry
... In modern industrial organic chemistry a new compound is produced by converting one compound into another. ...
... In modern industrial organic chemistry a new compound is produced by converting one compound into another. ...
Carboxylic Acid
... You know Alkanes and Benzenes, and Alkynes and Alkenes, Amines and Alcohols, Aldehydes and Ketones...... But do you recall, the most famous functional group of all Carboxylic Acid, Has a carbonyl and a hydroxyl It loves to donate protons Then an anion is formed Of all the other acids It’s the most c ...
... You know Alkanes and Benzenes, and Alkynes and Alkenes, Amines and Alcohols, Aldehydes and Ketones...... But do you recall, the most famous functional group of all Carboxylic Acid, Has a carbonyl and a hydroxyl It loves to donate protons Then an anion is formed Of all the other acids It’s the most c ...
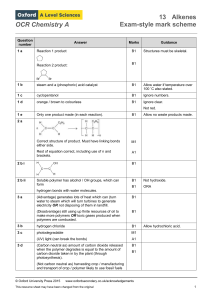
OCR Chemistry A Question number Answer Marks Guidance 1 a
... version and can be displayed or skeletal or a mixture, as long as they are unambiguous. ...
... version and can be displayed or skeletal or a mixture, as long as they are unambiguous. ...
Chapter 25 Organic and Biological Chemistry
... 1. Find the longest chain in the molecule. 2. Number the chain from the end nearest the first substituent encountered. 3. List the substituents as a prefix along with the number(s) of the carbon(s) to which they are attached. ...
... 1. Find the longest chain in the molecule. 2. Number the chain from the end nearest the first substituent encountered. 3. List the substituents as a prefix along with the number(s) of the carbon(s) to which they are attached. ...
Chapter 8 Alkenes and Alkynes II
... Modern Statement of Markovnikov’s Rule: In the ionic addition of an unsymmetrical reagent to a double bond, the positive portion of the adding reagent attaches itself to a carbon atom of the double bond so as to yield the more stable carbocation as an intermediate ...
... Modern Statement of Markovnikov’s Rule: In the ionic addition of an unsymmetrical reagent to a double bond, the positive portion of the adding reagent attaches itself to a carbon atom of the double bond so as to yield the more stable carbocation as an intermediate ...
Zumd22
... products, geological and elemental carbon is inorganic carbon. Other carbon-containing molecules are organic by virtue of carbon’s presence. There are no end to the combinatorial possibilities since C bonds to C! ...
... products, geological and elemental carbon is inorganic carbon. Other carbon-containing molecules are organic by virtue of carbon’s presence. There are no end to the combinatorial possibilities since C bonds to C! ...
Chapter 7
... sodium ethoxide in ethanol, it reacts rapidly; the product is 4-tert-butylcyclohexene. Under the same condition , trans1-bromo-4-ter-butylcyclohexane reacts very slowly. Write conformational structures and explain the difference in reactivity of these cis-trans isomers. ...
... sodium ethoxide in ethanol, it reacts rapidly; the product is 4-tert-butylcyclohexene. Under the same condition , trans1-bromo-4-ter-butylcyclohexane reacts very slowly. Write conformational structures and explain the difference in reactivity of these cis-trans isomers. ...
Alkene

In organic chemistry, an alkene is an unsaturated hydrocarbon that contains at least one carbon–carbon double bond. Alkene, olefin, and olefine are used often interchangeably (see nomenclature section below). Acyclic alkenes, with only one double bond and no other functional groups, known as mono-enes, form a homologous series of hydrocarbons with the general formula CnH2n. Alkenes have two hydrogen atoms less than the corresponding alkane (with the same number of carbon atoms). The simplest alkene, ethylene (C2H4), which has the International Union of Pure and Applied Chemistry (IUPAC) name ethene is the organic compound produced on the largest scale industrially. Aromatic compounds are often drawn as cyclic alkenes, but their structure and properties are different and they are not considered to be alkenes.