* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download OCR Chemistry A Question number Answer Marks Guidance 1 a
Survey
Document related concepts
Transcript
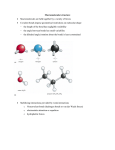
13 Alkenes Exam-style mark scheme OCR Chemistry A Question number 1a Answer Marks Guidance Reaction 1 product B1 Structures must be skeletal. Reaction 2 product: B1 1b steam and a (phosphoric) acid catalyst B1 Allow water if temperature over 100 ˚C also stated. 1c cyclopentanol B1 Ignore numbers. 1d orange / brown to colourless B1 Ignore clear. Not red. 1e Only one product made (in each reaction). B1 Correct structure of product. Must have linking bonds either side. M1 Rest of equation correct, including use of n and brackets. A1 Allow no waste products made. 2a 2bi 2 b ii B1 Soluble polymer has alcohol / OH groups, which can form B1 Not hydroxide. B1 ORA hydrogen bonds with water molecules. 3a (Advantage) generates lots of heat which can (turn water to steam which will turn turbines to generate electricity OR not disposing of them in landfill. B1 (Disadvantage) still using up finite resources of oil to make more polymers OR toxic gases produced when polymers are combusted. B1 3b hydrogen chloride B1 3c photodegradable M1 (UV) light (can break the bonds) A1 (Carbon neutral as) amount of carbon dioxide released when the polymer degrades is equal to the amount of carbon dioxide taken in by the plant (through photosynthesis). B1 3d Allow hydrochloric acid. B1 (Not carbon neutral as) harvesting crop / manufacturing and transport of crop / polymer likely to use fossil fuels © Oxford University Press 2015 www.oxfordsecondary.co.uk/acknowledgements This resource sheet may have been changed from the original 1 13 Alkenes Exam-style mark scheme OCR Chemistry A Question number Answer Marks Guidance (so more carbon dioxide emitted than absorbed). 4a 2-methylbut-2-ene B1 4b electrophilic M1 addition A1 Curly arrow from double bond to the H of H–Cl B1 Curly arrow from H–Cl bond to the Cl AND correct partial charges shown on the HCl B1 Positive charge on the correct carbon atom of the carbocation M1 Curly arrow from a lone pair of electrons OR the negative charge on the chloride ion to the positive carbon. C1 4d A tertiary carbocation is more stable than a secondary carbocation. B1 4e 2-chloro-3-methylbutane C1 4c ECF this mark if incorrect carbocation drawn. Allow 3-chloro-2-methylbutane. Allow ECF for 2-chloro-2-methyl if curly arrow mechanism produces minor product in part c. 4f Heterolytic M1 (Double headed) curly arrow shows the movement of a pair of electrons, so bond breaks when both electrons move to one of the bonded atoms. A1 Mark for showing overlap of two p-orbitals on the carbon atoms. B1 Mark for π-bond shown above and below the C–C bond. B1 5bi trigonal planar B1 5 b ii Each carbon atom is surrounded by three bonds / areas of electron density which repel equally. B1 5c (cannot exhibit E/Z because) each carbon of the CC double bond does not have 2 different groups attached to it. B1 5a © Oxford University Press 2015 www.oxfordsecondary.co.uk/acknowledgements This resource sheet may have been changed from the original 2 13 Alkenes Exam-style mark scheme OCR Chemistry A Question number Answer 5di 5 d ii Any 2 from: Marks Guidance B1 Must be skeletal. B1 Structures can be the E or Z version and can be displayed or skeletal or a mixture, as long as they are unambiguous. B1 Do not allow any structures which do not exhibit E/Z isomers, e.g. hex-1-ene, 2,3-dimethylbut-2-ene or 2-methylpent-2-ene. 5ei E, because the highest priority groups are opposite each other (across the CC double bond). Cl has a higher atomic number than C AND F has a higher atomic number than H. 5 e ii (In order to have cis or trans isomers) each C atom of the CC double bond must have two different substituent groups and one of those groups must be hydrogen. © Oxford University Press 2015 M1 Not just E. A1 B1 Allow AW. www.oxfordsecondary.co.uk/acknowledgements This resource sheet may have been changed from the original 3