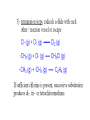* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Topic 10. Organic chemistry
Cracking (chemistry) wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Asymmetric induction wikipedia , lookup
Aromatization wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Aromaticity wikipedia , lookup
Homoaromaticity wikipedia , lookup
Physical organic chemistry wikipedia , lookup
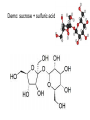
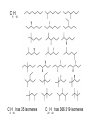
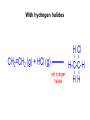
Topic 10. Organic chemistry BBC News: Complex organic molecule found in interstellar space ● Science reporter Michael Eyre: ”Scientists have found the beginnings of life-bearing chemistry at the centre of the galaxy.” https://www.youtube.com/watch?v=UloIw7dhnlQ&list=PLCmjFQPlyHZJlg9G0l3I7Plr0-BK3W-a6 Introduction ● ● Organic chemistry: The study of carboncontaining compounds with the exception of the allotropes of the element itself, metal carbonates and its oxides and halides. Organic compounds: Contain carbon and hydrogen. They may contain other elements too, such as oxygen, nitrogen, halogens or sulfur. Inorganic carbon compounds: ● carbon oxides, hydrogencarbonates, carbonates, cyanides ● Demo: sucrose + sulfuric acid Carbon is a unique element Carbon can form multiple bonds with itself and with atoms of other elements (single, double and triple bonds). ● ● The C-C bond is strong and thermally stable. ● Carbon atoms can also join to form rings. ● ● There are at least 6 million different organic compounds, all with their own physical and chemical properties. In order to study all these organic compounds, they are categorized into ”families” or homologous series. Homologous series ● ● ● A homologous series is one in which all the members have the same general formula. The neighbouring members of the series differ by only -CH2. The alkanes form a series of compounds that all have the general formula CnH2n+2 Some common homologous series Homologous series Functional group Alkanes Alkenes Halogenoalkanes Alcohols Aldehydes Ketones Carboxylic acids Amines Esters Aromatic compounds Benzene ● Each functional group has its own characteristic reactions. Formulas ● ● ● Empirical formula: The simplest whole number ratio of the atoms in a molecule. Molecular formula: The actual number of atoms in a molecule. Structural formula: How the atoms are bonded to each other in a molecule. Structural isomers ● Compounds with the same molecular formula but with different arrangements of atoms. ● C4H10 (2 isomers) ● C5H12 (3 isomers) ● C6H14 (4 isomers) C8H18 C9H20 has 35 isomeres C20H42 has 366 319 isomeres Naming organic compounds Prefix Where are the substituents? - Parent How many carbons? - Suffix What family? e.g. 3-methylhexane 1. Identify the longest carbon chain (containing the functional group). Number of carbons 1 2 3 4 5 6 Stem in IUPAC name methethpropbutpenthex- 2. Identify the type of bonding in the chain or ring ● Single bond: -an- ● Double bond: -en- ● Triple bond: -yn- 3. Identify the functional group/groups Homologous series Functional group alkane Suffix in IUPAC name -ane alkene double bond -ene alcohol hydroxyl group -ol aldehyde carbonyl -al ketone carbonyl -one carboxylic acid carboxyl -oic acid halogenoalkane 4. Identify the side chains or substituent groups Side chain/substituent group Prefix methylethylpropylbutylfluoro-, chloro-, bromo-, iodoaminohydroxy- ● If there are two or more functional groups in a molecule, the order is: carboxylic acid > ketone/aldehyde > alcohol > amine > halide ● If there are more than one substituent group of the same type, the following prefixes are used: ● mono- ● di- ● tri- ● tetra- 4. Numbers are used to give the position of groups or bonds ● The carbon with the functional group should have the lowest possible number. Ex. Draw the structures and give the IUPAC names for all non-cyclic isomers of: Alkanes C-1 to C-6 Straight chain alkenes C-2 to C-6 Primary, secondary and tertiary carbon atoms ● Primary: ● Secondary: ● Tertiary: The effect of the functional group on the volatility ● The stronger the intermolecular forces, the higher the boiling point. Trends in boiling points ● As the carbon chain gets longer (= the mass of the molecule increases) the van der Waals´forces of attraction increases. ● Molecules with branches are more spherical in shape, which reduces the contact area between them and lowers their boiling points. The effect of the functional group on the solubility in water ● ● The greater the polarity of the functional group, the more soluble in water the compound is. As the size of the hydrocarbon chain increases, the solubility decreases. ● Small molecules of alcohols, amines and carboxylic acids are all water soluble and form hydrogen bonds. e.g. ethanol dissolves in water, but hexan-1-ol is only slightly soluble. ● ● ● Aldehydes, ketones and esters are less soluble in water. Halogenoalkanes, alkanes and alkenes do not dissolve in water, but are soluble in other non-polar solvents. Propan-1-ol is a good solvent because it can dissolve both polar and non-polar substances. Crude oil ● ● Crude oil was formed over millions of years ago when the remains of animals and plants were trapped under layers of rock. Crude oil is a complex mixture of many different organic compounds, mainly alkanes. ● In an oil refinery the alkanes are separated (according to boiling point) by fractional distillation. ● The various fractions, with different physical characteristics, are used in a wide variety of circumstances, mainly as fuels. The molecules in the higher boiling fractions are broken down (=cracked) in the presence of a catalyst to give smaller more useful molecules. ● For example alkenes can be obtained in large quantities from crude oil. ● Alkanes ● ● ● Alkanes are saturated hydrocarbons (= they contain only single C-C bonds) . Hydrocarbon: Compounds that ONLY contain hydrogen and carbon. General formula: CnH2n+2 Reactivity ● ● Alkanes are stable under most conditions, because of strong C-C and C-H bonds and low polarity. They only undergo combustion reactions with oxygen and substitution reactions with halogens. a) Combustion Ex. Give a balanced equation for the combustion reaction of gasoline (C8H18) in excess oxygen. ● The combustion reaction is highly exothermic, as the bond enthalpies of the products are greater than those of the reactants. b) Substitution Alkenes General formula: CnH2n ● ● ● The double bond is made up of two different bonds: The pi-bond is a weaker bond than the sigma-bond and it is relatively easily broken. Addition reactions: With hydrogen = hydrogenation With halogens = halogenation With hydrogen halides With water = hydration Addition polymerization Polyethene Polypropene Polychloroethene Alkanes vs. alkenes ● ● ● ● Alkenes have a higher ratio of carbon to hydrogen than alkanes and will burn with a much more dirty and smoky flame (because they contain more unborn carbon). Aromatic compounds burn with an even smokier flame. Alkenes readily undergo addition reactions, whereas alkanes will not. Demo: Alkene test Alcohols a) Combustion ● ● ● Like the hydrocarbons, alcohols burn in oxygen with the release of large amounts of energy. Alcohols are therefore an important source of fuel. Alcohols release less energy than hydrocarbons with similar molar mass: b) Oxidation KCr2O7 An aldehyde is obtainded if the product is distilled off as it is formed. A carboxylic acid is obtained if the mixture is heated under reflux. Halogenoalkanes Substitution reaction Reaction pathways In modern industrial organic chemistry a new compound is produced by converting one compound into another. ● Important organic compounds are for example: polymers, pharmaceuticals, dyes and solvents. ● The oil industry is the main source of the raw materials for the organic compounds that are produced. ● Reaction pathways ● ● The starting compound has to be converted into the desired product using as few steps as possible and each step should have the highest possible yield. The reaction pathway describes this sequence of several steps between the starting material and the product. Ex. 1 Devise two-step syntheses of the following products from the starting material listed. Include any necessary experimental conditions and an equation for each step. a) Ethanoic acid from ethene b) Butan-1-ol from butane c) Hexan-3-one from 3-bromohexane d) 1,2,3-trichloropropane from propene e) Propanal from 1-bromopropane Ex. 2 The synthesis of a particular organic product involves seven separate stages. If each stage produces a yield of 70%, calculate the percentage yield of the final product.