Metabolism - rci.rutgers.edu
... In most energy conversions, energy is lost as heat Entropy may be defined as an increase in disorder or randomness Second law of thermodynamics a. Total amount of entropy (S) increases in the universe as energy is converted from one form to another ...
... In most energy conversions, energy is lost as heat Entropy may be defined as an increase in disorder or randomness Second law of thermodynamics a. Total amount of entropy (S) increases in the universe as energy is converted from one form to another ...
COMPARATIVE MODELING AND MOLECULAR
... the evolutionary pressure on the organization of protein biosynthetic machinery. This ubiquitous assemblage consists of 11 polypeptide subunits. This complex comprises 9 aminoacyl-tRNA synthetases particular for their corresponding amino acid of class I and class II tRNA synthetases, that are monome ...
... the evolutionary pressure on the organization of protein biosynthetic machinery. This ubiquitous assemblage consists of 11 polypeptide subunits. This complex comprises 9 aminoacyl-tRNA synthetases particular for their corresponding amino acid of class I and class II tRNA synthetases, that are monome ...
Cellular Respiration Note Packet
... C. There is much _____________ stored in this molecule of _______________. This energy must be released in ___________________________ steps. If all the energy from glucose were released at once, most of it would be lost as ______________________. The energy stored in glucose will be released bit by ...
... C. There is much _____________ stored in this molecule of _______________. This energy must be released in ___________________________ steps. If all the energy from glucose were released at once, most of it would be lost as ______________________. The energy stored in glucose will be released bit by ...
Cellular Respiration and Photosynthesis
... Inner membrane proteins reduced by NADH and FADH2 AcetylCoA broken down further, releasing two CO2 molecules; C electrons and H sequestered by NADH and FADH2 Oxygen reduced by electrons from inner membrane proteins; D binds with 2 protons and released as waste H2O E Glucose hydrolyzed into two pyruv ...
... Inner membrane proteins reduced by NADH and FADH2 AcetylCoA broken down further, releasing two CO2 molecules; C electrons and H sequestered by NADH and FADH2 Oxygen reduced by electrons from inner membrane proteins; D binds with 2 protons and released as waste H2O E Glucose hydrolyzed into two pyruv ...
Document
... • The simplest of the purines, adenosine is found in biological fluids throughout the body. • Adenosine differs from ATP in that it is not stored by and released from secretory vesicles. • Rather, it exists free in the cytosol of all cells and is transported in and out of cells mainly via a membrane ...
... • The simplest of the purines, adenosine is found in biological fluids throughout the body. • Adenosine differs from ATP in that it is not stored by and released from secretory vesicles. • Rather, it exists free in the cytosol of all cells and is transported in and out of cells mainly via a membrane ...
2. Citric acid cycle
... Compare and contrast aerobic respiration and fermentation for three things that are similar/shared AND three things that are different! ...
... Compare and contrast aerobic respiration and fermentation for three things that are similar/shared AND three things that are different! ...
Stryer An overview of the citric acid cycle
... Origin of mitochondria: the endosymbiont hypothesis The endosymbiont hypothesis suggests that mitochondria have evolved from anaerobic bacteria which were phagocytosed by eukaryote cells at the time oxygen appeared on earth, Similarities between mitochondria and bacteria include the presence of: • ...
... Origin of mitochondria: the endosymbiont hypothesis The endosymbiont hypothesis suggests that mitochondria have evolved from anaerobic bacteria which were phagocytosed by eukaryote cells at the time oxygen appeared on earth, Similarities between mitochondria and bacteria include the presence of: • ...
Chapter 5 Gases
... Acetyl–CoA formation and the Krebs cycle in the mitochondrial matrix break down the pyruvate to CO2, which leaves the cell. Ten additional coenzymes are reduced. Two ATP form. Stage 3 In electron transfer phosphorylation, the reduced coenzymes give up electrons and hydrogen ions to electron transfer ...
... Acetyl–CoA formation and the Krebs cycle in the mitochondrial matrix break down the pyruvate to CO2, which leaves the cell. Ten additional coenzymes are reduced. Two ATP form. Stage 3 In electron transfer phosphorylation, the reduced coenzymes give up electrons and hydrogen ions to electron transfer ...
here - Sites@PSU
... Lactococcus sp. Lactobacillus sp. Leuconostoc sp. Pediococcus sp. Oenococcus sp. Streptococcus sp. Enterococcus sp. Sporolactobacillus sp. Carnobacterium sp. Aerococcus sp. Tetragenococcus sp. Vagococcus sp. Weisella sp. ...
... Lactococcus sp. Lactobacillus sp. Leuconostoc sp. Pediococcus sp. Oenococcus sp. Streptococcus sp. Enterococcus sp. Sporolactobacillus sp. Carnobacterium sp. Aerococcus sp. Tetragenococcus sp. Vagococcus sp. Weisella sp. ...
respiration revision quiz
... ATP can be …………………….. to form ADP and Pi (……………………………..), releasing 30.6 KJ.mol-‐1 of energy. It is a …………………………….-‐lived molecule which is constantly being hydrolysed and resynthesized. ATP + H20 à AD ...
... ATP can be …………………….. to form ADP and Pi (……………………………..), releasing 30.6 KJ.mol-‐1 of energy. It is a …………………………….-‐lived molecule which is constantly being hydrolysed and resynthesized. ATP + H20 à AD ...
Exam 2 - Saddleback College
... • know the factors that influence enzymatic activity including feedback regulation • be able to tie in: anabolic/catabolic with endergonic/exergonic, simple/complex, making energy/using energy • Photosynthesis (Ch 5) – as discussed with the chloroplast in chapter 4 and the mini lab lecture • Make su ...
... • know the factors that influence enzymatic activity including feedback regulation • be able to tie in: anabolic/catabolic with endergonic/exergonic, simple/complex, making energy/using energy • Photosynthesis (Ch 5) – as discussed with the chloroplast in chapter 4 and the mini lab lecture • Make su ...
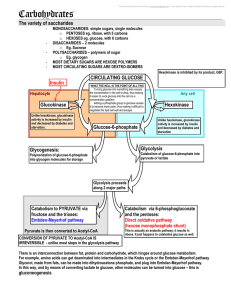
5 carbohydrates and the Krebs Cycle
... FRUCTOSE is converted to fructose-6-phosphate, analogous process to the phosphorylation of glucose; its even mediated by the same enzyme (hexokinase). The reaction of fructose phosphorylation can occur in the ABSENCE of insulin; but most of this occurs in the intestine, so it is not especially great ...
... FRUCTOSE is converted to fructose-6-phosphate, analogous process to the phosphorylation of glucose; its even mediated by the same enzyme (hexokinase). The reaction of fructose phosphorylation can occur in the ABSENCE of insulin; but most of this occurs in the intestine, so it is not especially great ...
Cellular Respiration Name: Period: ______ Date: 1. Define cellular
... 33. What is the function of the electron transport chain? ___________________________________________________ 34. Where is the electron transport chain located in eukaryotes? _____________________________________________ 35. Where is the electron transport chain located in prokaryotes? _____________ ...
... 33. What is the function of the electron transport chain? ___________________________________________________ 34. Where is the electron transport chain located in eukaryotes? _____________________________________________ 35. Where is the electron transport chain located in prokaryotes? _____________ ...
3.2 and 3.3
... Monomer of Nucleic acids are….. Name the three groups in one monomer… Nucleic acids primary function is to …… What process puts these monomers together to form long chains…. • What process breaks down ATP for energy….. ...
... Monomer of Nucleic acids are….. Name the three groups in one monomer… Nucleic acids primary function is to …… What process puts these monomers together to form long chains…. • What process breaks down ATP for energy….. ...
Chapter 32 - How Animals Harvest Energy Stored in Nutrients
... Animals require a constant supply of energy to perform biological work. The energy-rich molecule ATP usually provides this energy. All animals can generate ATP by breaking down organic nutrients (carbohydrates, fats, and proteins). The energy released is used to join ADP and phosphate (Pi) to form A ...
... Animals require a constant supply of energy to perform biological work. The energy-rich molecule ATP usually provides this energy. All animals can generate ATP by breaking down organic nutrients (carbohydrates, fats, and proteins). The energy released is used to join ADP and phosphate (Pi) to form A ...
Chapter 16
... 14. Succinate dehydrogenase is the only membrane-bound citric acid enzyme since the covalently bound FADH2 is only oxidized by the electron transport chain reaction. 15. Although the oxaloacetate formation form L-malate is relatively high endergonic reaction, this reaction occurs, because: 1. The [o ...
... 14. Succinate dehydrogenase is the only membrane-bound citric acid enzyme since the covalently bound FADH2 is only oxidized by the electron transport chain reaction. 15. Although the oxaloacetate formation form L-malate is relatively high endergonic reaction, this reaction occurs, because: 1. The [o ...
Chapter 7
... respiration requires O2, it is called an aerobic process. c. What happens to glucose after it’s been split up and depleted of its energy? Well, remember that glucose is C6H12O6. When it is split up, its Cs and Os stick together to form CO2 (carbon dioxide). It’s Hs join up with the O2 provided by yo ...
... respiration requires O2, it is called an aerobic process. c. What happens to glucose after it’s been split up and depleted of its energy? Well, remember that glucose is C6H12O6. When it is split up, its Cs and Os stick together to form CO2 (carbon dioxide). It’s Hs join up with the O2 provided by yo ...
this lecture as PDF here
... ~54 kcal/mole (from delta Go' for oxidation), then to make glucose costs 780 kcal/mole, more than the energy available by oxidizing glucose. • Conclusion: making sugar is expensive! Cell needs to supply large quantities of ATP and NADPH. ...
... ~54 kcal/mole (from delta Go' for oxidation), then to make glucose costs 780 kcal/mole, more than the energy available by oxidizing glucose. • Conclusion: making sugar is expensive! Cell needs to supply large quantities of ATP and NADPH. ...
ILS Unit 6 Semester 2 Name Teacher
... ILS—Laboratory—Cellular Respiration of Yeast Purpose: To observe how added sugar (a food source) affects the cellular respiration of yeast. ...
... ILS—Laboratory—Cellular Respiration of Yeast Purpose: To observe how added sugar (a food source) affects the cellular respiration of yeast. ...
Option B Rev A
... ATP yields assuming optimal function of ETS. In reality, however, electron leakage occurs. Abbreviations: ATP, adenosine triphosphate; CoA, coenzyme A; ETS, electron transport system; FADH 2, reduced form of flavin adenine dinucleotide; G-P, glucose to pyruvate; GTP, guanosine triphosphate; H+, hydr ...
... ATP yields assuming optimal function of ETS. In reality, however, electron leakage occurs. Abbreviations: ATP, adenosine triphosphate; CoA, coenzyme A; ETS, electron transport system; FADH 2, reduced form of flavin adenine dinucleotide; G-P, glucose to pyruvate; GTP, guanosine triphosphate; H+, hydr ...
Lect 1 (Metabolic Pathways) Lect 2 (Enzymes) Lect 3 (Glucose
... found only in the liver, doesn’t have endproduct inhibition, it functions at higher [glucose]. Glucokinase can be sequestered in the nucleus by Fructose 6-phosphate where a nuclear protein will bind it and released by glucose. Isoenzymes of hexokinase regulates glycolysis 2nd Futile Cycle Phosphofru ...
... found only in the liver, doesn’t have endproduct inhibition, it functions at higher [glucose]. Glucokinase can be sequestered in the nucleus by Fructose 6-phosphate where a nuclear protein will bind it and released by glucose. Isoenzymes of hexokinase regulates glycolysis 2nd Futile Cycle Phosphofru ...
Practice Exam 3 Answers
... Name the two enzymes that catalyze a reaction in which ATP is consumed? __________________________________________ Which enzyme catalyzes a reaction in which NADH is produced? _____________________ Which enzyme converts G3P into 1,3 BPG? __________________________ Name two enzyme reactions from glyc ...
... Name the two enzymes that catalyze a reaction in which ATP is consumed? __________________________________________ Which enzyme catalyzes a reaction in which NADH is produced? _____________________ Which enzyme converts G3P into 1,3 BPG? __________________________ Name two enzyme reactions from glyc ...
Unit 3 Biology Webquest/Book quest - Mandarin High School
... Textbook: p. 222 15. Identify the mitochondria as the cellular structure involved in respiration stressing that internal membranes are the primary site of reactions. Textbook: p. 222, 227, 228 16. Identify tissues in the body that require high concentration of mitochondria due to high energy require ...
... Textbook: p. 222 15. Identify the mitochondria as the cellular structure involved in respiration stressing that internal membranes are the primary site of reactions. Textbook: p. 222, 227, 228 16. Identify tissues in the body that require high concentration of mitochondria due to high energy require ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.