2.8 Respiration
... • Cell respiration is the controlled release of energy from organic compounds to produce ATP. • ATP from cell respiration is immediately available as a source of energy in the cell. • Anaerobic cell respiration gives a small yield of ATP from glucose. • Aerobic cell respiration requires oxygen and g ...
... • Cell respiration is the controlled release of energy from organic compounds to produce ATP. • ATP from cell respiration is immediately available as a source of energy in the cell. • Anaerobic cell respiration gives a small yield of ATP from glucose. • Aerobic cell respiration requires oxygen and g ...
09_Lectures_PPT
... passing through channels in ATP synthase • ATP synthase uses the exergonic flow of H+ to drive phosphorylation of ATP ...
... passing through channels in ATP synthase • ATP synthase uses the exergonic flow of H+ to drive phosphorylation of ATP ...
Sport`s Nutrition Slides
... minutes, glucose is broken down anaerobically through glycolysis. You can produce energy very fast this way, but not for long. Glucose is broken down to pyruvate, so when not enough oxygen is available, lactic acid forms. Lactic acid changes the pH in the muscle, which results in the burning sensati ...
... minutes, glucose is broken down anaerobically through glycolysis. You can produce energy very fast this way, but not for long. Glucose is broken down to pyruvate, so when not enough oxygen is available, lactic acid forms. Lactic acid changes the pH in the muscle, which results in the burning sensati ...
Can you describe the various methods of cell membrane transport?
... Electron micrograph of a human lymphocyte cell—a number of mitochondria are visible. ...
... Electron micrograph of a human lymphocyte cell—a number of mitochondria are visible. ...
Cell Structure
... • Energy Investment phase: active glucose w/ 2 ATP • Energy Pay Off phase: oxidation the removal of H, production of NADH, ATP • This makes 4 molecules of ATP ...
... • Energy Investment phase: active glucose w/ 2 ATP • Energy Pay Off phase: oxidation the removal of H, production of NADH, ATP • This makes 4 molecules of ATP ...
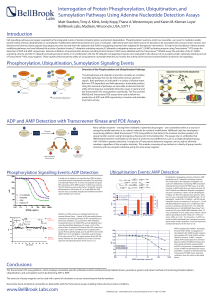
Interrogation of Protein Phosphorylation
... diseases and have thus been popular drug targets, but only recently have the ubiquitin-and SUMO-conjugating enzymes been targeted for therapeutic intervention. To help in the elucidation of these protein modifying pathways, we have followed the activity of protein kinases, E1 ubiquitin-activating en ...
... diseases and have thus been popular drug targets, but only recently have the ubiquitin-and SUMO-conjugating enzymes been targeted for therapeutic intervention. To help in the elucidation of these protein modifying pathways, we have followed the activity of protein kinases, E1 ubiquitin-activating en ...
Spring 2016 Practice Final Exam w/ solution
... c (6 pts). Koop and Lehninger are pioneering scientists whose work led to the elucidation of fatty acid catabolism. Succinctly describe Knoop’s and Lehninger’s experiments and the conclusions from these studies (limit to four sentences for each experiment). Koop’s Experiment: Fed dog with even- and ...
... c (6 pts). Koop and Lehninger are pioneering scientists whose work led to the elucidation of fatty acid catabolism. Succinctly describe Knoop’s and Lehninger’s experiments and the conclusions from these studies (limit to four sentences for each experiment). Koop’s Experiment: Fed dog with even- and ...
Link to Unit 4.0
... SC.912.L.18.7: Identify the reactants, products, and basic functions of photosynthesis. SC.912.L.18.8: Identify the reactants, products, and basic functions of aerobic and anaerobic cellular respiration. SC.912.L.18.9: Explain the interrelated nature of photosynthesis and cellular respiration. ...
... SC.912.L.18.7: Identify the reactants, products, and basic functions of photosynthesis. SC.912.L.18.8: Identify the reactants, products, and basic functions of aerobic and anaerobic cellular respiration. SC.912.L.18.9: Explain the interrelated nature of photosynthesis and cellular respiration. ...
AP Midterm Study Guide
... Matter: anything that has mass and takes up space Element: matter in its simplest form Compound: two or more elements combined in simple whole number ratios of atoms Atom: the smallest form of an element that still displays its particular properties Consists of a nucleus of positively charged prot ...
... Matter: anything that has mass and takes up space Element: matter in its simplest form Compound: two or more elements combined in simple whole number ratios of atoms Atom: the smallest form of an element that still displays its particular properties Consists of a nucleus of positively charged prot ...
Cellular Respiration and Photosynthesis
... From the blood-borne substrate pool. | Glucose -monosaccharide sugar -- | You're sweeter than a woman's kiss | 'Cause I need you for glycolysis. | I just can't believe the way my muscles take you in. | (For you, they'll open the door.) | All it takes is a little bit of insulin | (To upregulate GLUT4 ...
... From the blood-borne substrate pool. | Glucose -monosaccharide sugar -- | You're sweeter than a woman's kiss | 'Cause I need you for glycolysis. | I just can't believe the way my muscles take you in. | (For you, they'll open the door.) | All it takes is a little bit of insulin | (To upregulate GLUT4 ...
KREBS CYCLE Definition Krebs cycle (aka tricarboxylic acid cycle
... 5. Second oxidative-decarboxylation takes place. α-ketoglutarate is converted to succinyl-CoA. CO2 and NADH are produced. ...
... 5. Second oxidative-decarboxylation takes place. α-ketoglutarate is converted to succinyl-CoA. CO2 and NADH are produced. ...
Review Take Home
... Cellular Respiration Equations = C6H12O6 + 6O2 6CO2 + 6H2O + Energy Note that not all of the energy released from glucose by cellular respiration is captured in ATP: some of the energy is converted to heat. *To use energy from food: Cellular respiration transfers energy in organic molecules such a ...
... Cellular Respiration Equations = C6H12O6 + 6O2 6CO2 + 6H2O + Energy Note that not all of the energy released from glucose by cellular respiration is captured in ATP: some of the energy is converted to heat. *To use energy from food: Cellular respiration transfers energy in organic molecules such a ...
BIOL 202
... Ð Ð in early evolution of eukaryotic cells mitochondria and chloroplasts were bacteria engulfed by larger cells Ð Ð similar to bacteria Ð Ð double membrane (from phagocytosis) Ð Ð they have their own circular DNA (as in ...
... Ð Ð in early evolution of eukaryotic cells mitochondria and chloroplasts were bacteria engulfed by larger cells Ð Ð similar to bacteria Ð Ð double membrane (from phagocytosis) Ð Ð they have their own circular DNA (as in ...
For lecture notes click here
... formation of citric acid from acetyl-CoA and oxaloacetic acid is the first step in TCA the cycle removes H+ from organic molecules and transfer them to coenzymes in TCA cycle, the two-carbon acetyl group carried by CoA is attached to a four-carbon oxaloacetic acid molecule to make the six-carb ...
... formation of citric acid from acetyl-CoA and oxaloacetic acid is the first step in TCA the cycle removes H+ from organic molecules and transfer them to coenzymes in TCA cycle, the two-carbon acetyl group carried by CoA is attached to a four-carbon oxaloacetic acid molecule to make the six-carb ...
Anabolism
... ATP • Hydrogen atoms are released • Hydrogen atoms bind to NAD+ to produce NADH • NADH delivers hydrogen atoms to electron transport system if oxygen is available • ADP is phosphorylated to become ATP • Two molecules of pyruvic acid are produced • Two molecules of ATP are ...
... ATP • Hydrogen atoms are released • Hydrogen atoms bind to NAD+ to produce NADH • NADH delivers hydrogen atoms to electron transport system if oxygen is available • ADP is phosphorylated to become ATP • Two molecules of pyruvic acid are produced • Two molecules of ATP are ...
Ch 6 Metabolism_ Energy and Enzymes
... Hydrogen Peroxide is broken down by the enzyme catalase within cells. Potatoes hold the enzyme catalase, which will speed up the breakdown of hydrogen ...
... Hydrogen Peroxide is broken down by the enzyme catalase within cells. Potatoes hold the enzyme catalase, which will speed up the breakdown of hydrogen ...
CHAPTER 7 – COENZYMES AND VITAMINS CHAPTER SUMMARY
... 35. Ubiquinone (coenzyme ___) is lipid soluble and synthesized by almost all species. Its long hydrophobic chain allows it to dissolve into _______________, and its function is the transport of _______________ between membrane-embedded enzyme complexes. 36. Coenzyme Q is responsible for moving _____ ...
... 35. Ubiquinone (coenzyme ___) is lipid soluble and synthesized by almost all species. Its long hydrophobic chain allows it to dissolve into _______________, and its function is the transport of _______________ between membrane-embedded enzyme complexes. 36. Coenzyme Q is responsible for moving _____ ...
Marine Mammal Dive Response
... electron transport chain and eventually make water. Meanwhile, the hydrogen ions are pumped out into the intermembrane space. When they cross back into the mitochondrial matrix, they produce ATP. ...
... electron transport chain and eventually make water. Meanwhile, the hydrogen ions are pumped out into the intermembrane space. When they cross back into the mitochondrial matrix, they produce ATP. ...
Supplemental notes in pdf
... Life on earth is made possible by the biochemical reactions of photosynthesis, carbon fixation and aerobic respiration which together convert solar energy into ATP (and NADPH) which is used to synthesize carbohydrates from CO2 and H2O. Aerobic organisms, such as ourselves, consume carbohydrates as a ...
... Life on earth is made possible by the biochemical reactions of photosynthesis, carbon fixation and aerobic respiration which together convert solar energy into ATP (and NADPH) which is used to synthesize carbohydrates from CO2 and H2O. Aerobic organisms, such as ourselves, consume carbohydrates as a ...
Glycolysis
... *Values in this table from D. Voet & J. G. Voet (2004) Biochemistry, 3rd Edition, John ...
... *Values in this table from D. Voet & J. G. Voet (2004) Biochemistry, 3rd Edition, John ...
Q26to35
... D. The liver cannot convert fructose into glucose F1P to triose phosphates, back up gluconeogenesis ...
... D. The liver cannot convert fructose into glucose F1P to triose phosphates, back up gluconeogenesis ...
Reading Guide
... 27. Aromatic amino acids are both keto- and glucogenic because they are broken down into ___________________ and either ______________ or _______________. 28. Why is excess nitrogen from metabolic processes not simply excreted as ammonia? 29. What is glutamate’s particular role in nitrogen eliminat ...
... 27. Aromatic amino acids are both keto- and glucogenic because they are broken down into ___________________ and either ______________ or _______________. 28. Why is excess nitrogen from metabolic processes not simply excreted as ammonia? 29. What is glutamate’s particular role in nitrogen eliminat ...
Nutrition & Metabolism
... Acetyl CoA + Oxalocetic Acid Citric Acid Isocitric Acid CO2 NADH2 alpha-Ketoglutaric Acid CO2 NADH2 ...
... Acetyl CoA + Oxalocetic Acid Citric Acid Isocitric Acid CO2 NADH2 alpha-Ketoglutaric Acid CO2 NADH2 ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.