Food Utilization
... • Ingested chemical used for growth, repair or maintenance • Macronutrients • Micronutrients • Recommended daily allowances (RDA) – safe estimate of daily intake for standard needs • Essential nutrients can not be synthesized – minerals, vitamins, 8 amino acids and 1-3 fatty acids ...
... • Ingested chemical used for growth, repair or maintenance • Macronutrients • Micronutrients • Recommended daily allowances (RDA) – safe estimate of daily intake for standard needs • Essential nutrients can not be synthesized – minerals, vitamins, 8 amino acids and 1-3 fatty acids ...
Carbohydrates
... • Polymer (chain) of amino acids which are bonded together through covalent bonds called peptide bonds • 20 different amino acids are used to create proteins by the body – 11 of the 20 amino acids (nonessential) can be synthesized within the body and therefore do not need to be supplied by the diet ...
... • Polymer (chain) of amino acids which are bonded together through covalent bonds called peptide bonds • 20 different amino acids are used to create proteins by the body – 11 of the 20 amino acids (nonessential) can be synthesized within the body and therefore do not need to be supplied by the diet ...
23 Metabolism and Energy Production
... In the electron transport system, The electron carriers are attached to the inner membrane of the mitochondrion. There are four protein complexes: Complex I NADH dehydrogenase ...
... In the electron transport system, The electron carriers are attached to the inner membrane of the mitochondrion. There are four protein complexes: Complex I NADH dehydrogenase ...
2.-lactic-acid-metabolism
... must be present for glycolysis to continue). But as more and more lactic acid builds up, muscle fatigue is caused and an oxygen debt is created. ...
... must be present for glycolysis to continue). But as more and more lactic acid builds up, muscle fatigue is caused and an oxygen debt is created. ...
Lecture 6 Photosynthesis and Cellular Respiration
... 1. Plants and animals exchange materials through the processes of photosynthesis and respiration. Which of these statments is true about the way these two processes are related? A. The products of photosynthesis inhibit respiration. B. The products of photosythesis are also the products of respi ...
... 1. Plants and animals exchange materials through the processes of photosynthesis and respiration. Which of these statments is true about the way these two processes are related? A. The products of photosynthesis inhibit respiration. B. The products of photosythesis are also the products of respi ...
1 Glycolysis and carbon-carbon bond chemistry I. Intro to Glycolysis
... However, organisms had been photosynthesizing since at least a thousand million years before that. What happened to the O2 those early organisms produced? One idea holds that the evolved oxygen was complexed with iron to form ferric oxide; when the iron was saturated with oxygen, it began to appear ...
... However, organisms had been photosynthesizing since at least a thousand million years before that. What happened to the O2 those early organisms produced? One idea holds that the evolved oxygen was complexed with iron to form ferric oxide; when the iron was saturated with oxygen, it began to appear ...
Energy 1
... ATP is the only source of energy recognized by the cells Only a small amount of ATP is stored inside the muscle cells ...
... ATP is the only source of energy recognized by the cells Only a small amount of ATP is stored inside the muscle cells ...
Anaerobic Respiration
... • Anaerobic conditions mean that there is no final hydrogen acceptor at the end of chemiosmosis. • Because there is no oxygen, NAD and FAD are not regenerated, which results in oxidation being blocked (NAD and FAD can’t get rid of H). • This subsequently means that no further link reaction, Krebs cy ...
... • Anaerobic conditions mean that there is no final hydrogen acceptor at the end of chemiosmosis. • Because there is no oxygen, NAD and FAD are not regenerated, which results in oxidation being blocked (NAD and FAD can’t get rid of H). • This subsequently means that no further link reaction, Krebs cy ...
Anaerobic Respiration
... • Anaerobic conditions mean that there is no final hydrogen acceptor at the end of chemiosmosis. • Because there is no oxygen, NAD and FAD are not regenerated, which results in oxidation being blocked (NAD and FAD can’t get rid of H). • This subsequently means that no further link reaction, Krebs cy ...
... • Anaerobic conditions mean that there is no final hydrogen acceptor at the end of chemiosmosis. • Because there is no oxygen, NAD and FAD are not regenerated, which results in oxidation being blocked (NAD and FAD can’t get rid of H). • This subsequently means that no further link reaction, Krebs cy ...
A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid
... -some enzymes use coenzymes: thiamine, biotin, pyridoxamine, or tetrahydrofolate as additional nucleophilic reagents (b) Electrophilic Catalysis--numerous enzymes use bound metal ions to form complexes with substrates -metal ion functions as an electrophilic group -eg., carbonic anhydrase--contains ...
... -some enzymes use coenzymes: thiamine, biotin, pyridoxamine, or tetrahydrofolate as additional nucleophilic reagents (b) Electrophilic Catalysis--numerous enzymes use bound metal ions to form complexes with substrates -metal ion functions as an electrophilic group -eg., carbonic anhydrase--contains ...
Practice Q Ch 8 metabolism with key
... a. The reaction is endergonic and thus makes free energy available to fuel life processes b. The reaction requires free energy and thus is endergonic c. This is an exergonic reaction which is spontaneous and makes energy available d. The reaction requires free energy and is exergonic 15. Enzymes inf ...
... a. The reaction is endergonic and thus makes free energy available to fuel life processes b. The reaction requires free energy and thus is endergonic c. This is an exergonic reaction which is spontaneous and makes energy available d. The reaction requires free energy and is exergonic 15. Enzymes inf ...
Chapter 26
... • Energy has been lost as heat, stored in 2 ATP, 8 reduced NADH, 2 FADH2 molecules of the matrix reactions and 2 NADH from glycolysis • Citric acid cycle is also a source of substances for the synthesis of fats & nonessential amino acids ...
... • Energy has been lost as heat, stored in 2 ATP, 8 reduced NADH, 2 FADH2 molecules of the matrix reactions and 2 NADH from glycolysis • Citric acid cycle is also a source of substances for the synthesis of fats & nonessential amino acids ...
File - Wk 1-2
... released as ammonia. The most important ammoniaforming reaction, catalysed by glutamate dehydrogenase in liver and other tissues. This oxidative deamination/ reductive amination is freely reversible and can function both in the synthesis and degradation of glutamate. Either nicotinamide adenine dinu ...
... released as ammonia. The most important ammoniaforming reaction, catalysed by glutamate dehydrogenase in liver and other tissues. This oxidative deamination/ reductive amination is freely reversible and can function both in the synthesis and degradation of glutamate. Either nicotinamide adenine dinu ...
Metabolism - CSU, Chico
... Fatty acids are broken down two carbons at a time A typical fatty acid has the formula C18H34O2 Each two carbons go to Acetyl CoA which enters the TCA cycle and then Electron Transport Chain So a typical fatty acid spins the TCA cycle 9 times No wonder fats have so much energy associated with them g ...
... Fatty acids are broken down two carbons at a time A typical fatty acid has the formula C18H34O2 Each two carbons go to Acetyl CoA which enters the TCA cycle and then Electron Transport Chain So a typical fatty acid spins the TCA cycle 9 times No wonder fats have so much energy associated with them g ...
Metabolism: Dissimilatory (energy, catabolic) metabolism
... Fermentation to butyrate and to acetate ...
... Fermentation to butyrate and to acetate ...
Chapter 26
... • Carbon atoms of the glucose have all been carried away as CO2 and exhaled. • Energy has been lost as heat, stored in 2 ATP, 8 reduced NADH, 2 FADH2 molecules of the matrix reactions and 2 NADH from glycolysis • Citric acid cycle is also a source of substances for the synthesis of fats & nonessenti ...
... • Carbon atoms of the glucose have all been carried away as CO2 and exhaled. • Energy has been lost as heat, stored in 2 ATP, 8 reduced NADH, 2 FADH2 molecules of the matrix reactions and 2 NADH from glycolysis • Citric acid cycle is also a source of substances for the synthesis of fats & nonessenti ...
Energy Transformation — Cellular Respiration
... When physicians recognized that the breakdown of fats released ketone bodies, they were able to diagnose diseases such as diabetes and anorexia more easily, because people with these illnesses have bad breath. In starvation and severe diabetes mellitus, the body does not metabolize sugars properly, ...
... When physicians recognized that the breakdown of fats released ketone bodies, they were able to diagnose diseases such as diabetes and anorexia more easily, because people with these illnesses have bad breath. In starvation and severe diabetes mellitus, the body does not metabolize sugars properly, ...
Chapter 26
... • Carbon atoms of the glucose have all been carried away as CO2 and exhaled. • Energy has been lost as heat, stored in 2 ATP, 8 reduced NADH, 2 FADH2 molecules of the matrix reactions and 2 NADH from glycolysis • Citric acid cycle is also a source of substances for the synthesis of fats & nonessenti ...
... • Carbon atoms of the glucose have all been carried away as CO2 and exhaled. • Energy has been lost as heat, stored in 2 ATP, 8 reduced NADH, 2 FADH2 molecules of the matrix reactions and 2 NADH from glycolysis • Citric acid cycle is also a source of substances for the synthesis of fats & nonessenti ...
Classification of Enzymes - Lectures For UG-5
... “a” is the class, “b” is the subclass, “c” is the subsubclass, and “d” is the sub-sub-subclass. The “b” and “c” digits describe the reaction, while the “d” digit is used to distinguish between different enzymes of the same function based on the actual substrate in the reaction. • Example: for Alcoho ...
... “a” is the class, “b” is the subclass, “c” is the subsubclass, and “d” is the sub-sub-subclass. The “b” and “c” digits describe the reaction, while the “d” digit is used to distinguish between different enzymes of the same function based on the actual substrate in the reaction. • Example: for Alcoho ...
Two-Metal-Ion Catalysis in Adenylyl Cyclase
... conformational change from “open” to “closed.” However, a catalytic mechanism for the synthesis of cAMP was not obvious from the 2⬘-d-3⬘-AMP 䡠 PPi–inhibited structure. In particular, the active site lacked either an obvious catalytic base, which would abstract a proton from the 3⬘ hydroxyl of ATP, o ...
... conformational change from “open” to “closed.” However, a catalytic mechanism for the synthesis of cAMP was not obvious from the 2⬘-d-3⬘-AMP 䡠 PPi–inhibited structure. In particular, the active site lacked either an obvious catalytic base, which would abstract a proton from the 3⬘ hydroxyl of ATP, o ...
Short Answer Questions: a workshop
... The conversion of pyruvate to lactate does not yield enough ATP. Krebs cycle cannot take place as there is little acetyl CoA (from glucose there is none!) No reduced coenzymes to enter oxidative phosphorylation stage and so not enough ATP is made. Without ATP, cells (neurones, etc.) cannot carry out ...
... The conversion of pyruvate to lactate does not yield enough ATP. Krebs cycle cannot take place as there is little acetyl CoA (from glucose there is none!) No reduced coenzymes to enter oxidative phosphorylation stage and so not enough ATP is made. Without ATP, cells (neurones, etc.) cannot carry out ...
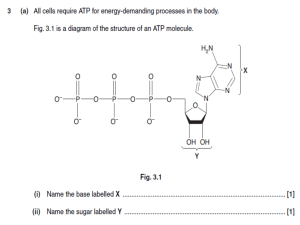
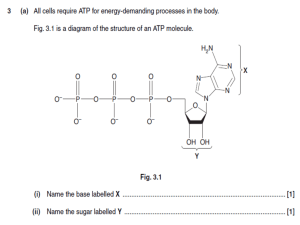
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.