Nitrate (NO3) + (e
... plasmid during conjugation Plasmid Small circular transferable DNA that contain extra genes; antibiotic resistance; metabolic ...
... plasmid during conjugation Plasmid Small circular transferable DNA that contain extra genes; antibiotic resistance; metabolic ...
Anaerobic Respiration - County Central High School
... VO2 max values vary between individuals and although you can increase yours through some training, it will decrease as you get older Even though VO2 max can be increased through exercise, lactic acid is also building up at an increased rate which can limit the amount of training and exercise an indi ...
... VO2 max values vary between individuals and although you can increase yours through some training, it will decrease as you get older Even though VO2 max can be increased through exercise, lactic acid is also building up at an increased rate which can limit the amount of training and exercise an indi ...
AP Bio - Semester 1 Review
... bonds is why this is the UNIVERSAL energy source (because it can be broken very quickly and releases lots of energy). Remember though, glucose has much more energy than ATP (1 glucose = 36 ATP). It’s just the glucose would take too long to break down to use immediately. o Phosphorylation – Removing ...
... bonds is why this is the UNIVERSAL energy source (because it can be broken very quickly and releases lots of energy). Remember though, glucose has much more energy than ATP (1 glucose = 36 ATP). It’s just the glucose would take too long to break down to use immediately. o Phosphorylation – Removing ...
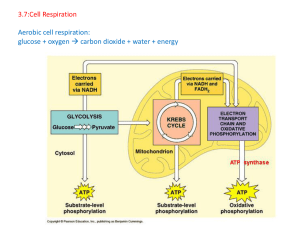
3.7:Cell Respiration Aerobic cell respiration: glucose
... rate can be measured by the disappearance of raw materials / 2 CO (in solution); rate of change of 2 CO can be measured (indirectly) by pH change; rate can be measured by the appearance of products/ 2 O /starch; rate can be measured by measuring rate of change of biomass; description of apparatus to ...
... rate can be measured by the disappearance of raw materials / 2 CO (in solution); rate of change of 2 CO can be measured (indirectly) by pH change; rate can be measured by the appearance of products/ 2 O /starch; rate can be measured by measuring rate of change of biomass; description of apparatus to ...
Transaminase. There are many types for each amino acid. They are
... Metabolism Lecture 11 — OXIDATIVE- & PHOTO-PHOSPHORYLATION — Restricted for students enrolled in MCB102, UC Berkeley, Spring 2008 ONLY ...
... Metabolism Lecture 11 — OXIDATIVE- & PHOTO-PHOSPHORYLATION — Restricted for students enrolled in MCB102, UC Berkeley, Spring 2008 ONLY ...
harvesting chemical energy
... oxidized states as they accept and donate electrons. Each component of the chain becomes reduced when it accepts electrons from its “uphill” neighbor, which is less electronegative. It then returns to its oxidized form as it passes electrons to its more electronegative ...
... oxidized states as they accept and donate electrons. Each component of the chain becomes reduced when it accepts electrons from its “uphill” neighbor, which is less electronegative. It then returns to its oxidized form as it passes electrons to its more electronegative ...
The Energy Requirement for Growth: An A ~ ~ lication of
... NADH yields 3 ATP, because the P/O ratios for the oxidationof FADH and NADH by the mitochondria1 respiratory chain are 2 and 3 , respectively. NADPH is valued at 4 ATP Eq because the energy-linked transhydrogenase, catalyzing the transfer of hydrogen from NADH t o NADP, requires an additional ATP (1 ...
... NADH yields 3 ATP, because the P/O ratios for the oxidationof FADH and NADH by the mitochondria1 respiratory chain are 2 and 3 , respectively. NADPH is valued at 4 ATP Eq because the energy-linked transhydrogenase, catalyzing the transfer of hydrogen from NADH t o NADP, requires an additional ATP (1 ...
09_DetailLectOut_jkAR
... oxidized states as they accept and donate electrons. Each component of the chain becomes reduced when it accepts electrons from its “uphill” neighbor, which is less electronegative. It then returns to its oxidized form as it passes electrons to its more electronegative ...
... oxidized states as they accept and donate electrons. Each component of the chain becomes reduced when it accepts electrons from its “uphill” neighbor, which is less electronegative. It then returns to its oxidized form as it passes electrons to its more electronegative ...
Exam 2 Material Outline MS Word
... 1. By 1920 – knew that genetic info resided on chromosomes but nobody could really say what a gene looked like and how it exactly worked 2. By 1930’s & 40’s knew that genes bring about the production of proteins 3. By 1940’s & 50’s knew that genes are composed of deoxyribonucleic acid (DNA) but did ...
... 1. By 1920 – knew that genetic info resided on chromosomes but nobody could really say what a gene looked like and how it exactly worked 2. By 1930’s & 40’s knew that genes bring about the production of proteins 3. By 1940’s & 50’s knew that genes are composed of deoxyribonucleic acid (DNA) but did ...
Question Report - FM Faculty Web Pages
... states that germs are the fundamental units of life states that germs can invade other organisms and cause disease states that germs will spontaneously arise from decaying meat is another name for the cell theory ...
... states that germs are the fundamental units of life states that germs can invade other organisms and cause disease states that germs will spontaneously arise from decaying meat is another name for the cell theory ...
chapter 9 cellular respiration: harvesting chemical
... In the electron transport chain, the electrons move from molecule to molecule until they combine with molecular oxygen and hydrogen ions to form water. As they are passed along the chain, the energy carried by these electrons is transformed in the mitochondrion into a form that can be used to synthe ...
... In the electron transport chain, the electrons move from molecule to molecule until they combine with molecular oxygen and hydrogen ions to form water. As they are passed along the chain, the energy carried by these electrons is transformed in the mitochondrion into a form that can be used to synthe ...
Chapter 9 – Cellular Respiration: Harvesting Chemical Energy
... As they are passed along the chain, the energy carried by these electrons is transformed in the mitochondrion into a form that can be used to synthesize ATP via oxidative phosphorylation. ...
... As they are passed along the chain, the energy carried by these electrons is transformed in the mitochondrion into a form that can be used to synthesize ATP via oxidative phosphorylation. ...
CHAPTER 6
... 10/4 = 2.5 for electrons entering as NADH For electrons entering as succinate (FADH2), about 6 H+ pumped per electron pair to oxygen 6/4 = 1.5 for electrons entering as succinate ...
... 10/4 = 2.5 for electrons entering as NADH For electrons entering as succinate (FADH2), about 6 H+ pumped per electron pair to oxygen 6/4 = 1.5 for electrons entering as succinate ...
ATP - Luzzago
... ATP synthase is the same in all living organisms. • It is a molecular motor with two parts: – F0 unit—the transmembrane H+ channel – F1 unit—projects into mitochondrial matrix; rotates to expose active sites for ATP synthesis ...
... ATP synthase is the same in all living organisms. • It is a molecular motor with two parts: – F0 unit—the transmembrane H+ channel – F1 unit—projects into mitochondrial matrix; rotates to expose active sites for ATP synthesis ...
BI0 120 cell and tissues
... B. The proton gradient established during electron transport is a form of potential energy. C. The electron transport chain can be found in the mitochondria of aerobic bacteria and other cells. D. The movement of protons down a concentration gradient is an endergonic process. E. ATP synthesis associ ...
... B. The proton gradient established during electron transport is a form of potential energy. C. The electron transport chain can be found in the mitochondria of aerobic bacteria and other cells. D. The movement of protons down a concentration gradient is an endergonic process. E. ATP synthesis associ ...
AP Biology Pre-Discussion Questions: Energy 5 - mhs
... What is the relationship between photosynthesis and aerobic cellular respiration? In cellular respiration, what is oxidized and what is reduced? What is the role of electron carrier molecules in energy processing systems? Why are they necessary? Is glucose the only molecule that can be catabolized d ...
... What is the relationship between photosynthesis and aerobic cellular respiration? In cellular respiration, what is oxidized and what is reduced? What is the role of electron carrier molecules in energy processing systems? Why are they necessary? Is glucose the only molecule that can be catabolized d ...
Ch13.doc
... Note: this is different from the answer in the text which was calculated from the reverse of the glycolytic pathway. c. Why are ΔGo’ and ΔG different? One is under standard conditions, the other, ΔG is the “actual’ ΔG in the conditions that either are artificial (in this case) or natural in the cell ...
... Note: this is different from the answer in the text which was calculated from the reverse of the glycolytic pathway. c. Why are ΔGo’ and ΔG different? One is under standard conditions, the other, ΔG is the “actual’ ΔG in the conditions that either are artificial (in this case) or natural in the cell ...
You will need to read on the aging process in your textbook
... • Fireflies use enzymes (luciferase) to produce light by bioluminescence • Researchers transferred genes for bioluminescence into strains of Salmonella so that the course of infection could be tracked by visualization ...
... • Fireflies use enzymes (luciferase) to produce light by bioluminescence • Researchers transferred genes for bioluminescence into strains of Salmonella so that the course of infection could be tracked by visualization ...
Selected Solutions to End of Chapter 13 Problems
... Note: this is different from the answer in the text which was calculated from the reverse of the glycolytic pathway. c. Why are ΔGo’ and ΔG different? One is under standard conditions, the other, ΔG is the “actual’ ΔG in the conditions that either are artificial (in this case) or natural in the cell ...
... Note: this is different from the answer in the text which was calculated from the reverse of the glycolytic pathway. c. Why are ΔGo’ and ΔG different? One is under standard conditions, the other, ΔG is the “actual’ ΔG in the conditions that either are artificial (in this case) or natural in the cell ...
03 - Respiration II, Photosynthesis I (ch.9,10) Sum13
... Cellular Respiration - anaerobic • no O2 – no oxidative phosphorylation ...
... Cellular Respiration - anaerobic • no O2 – no oxidative phosphorylation ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.