* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 2.Carbohydrates - Distance Education Chennai
Oxidative phosphorylation wikipedia , lookup
Signal transduction wikipedia , lookup
Isotopic labeling wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Genetic code wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Microbial metabolism wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
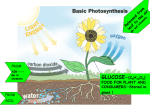
Photosynthesis wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Proteolysis wikipedia , lookup
Protein structure prediction wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Metalloprotein wikipedia , lookup
NUTRITIONAL BIOCHEMISTRY 5 MARK 1.DEFINE BIOCHEMISTRY Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. The laws of biochemistry govern all living organisms and living processes. By controlling information flow through biochemical signalling and the flow of chemical energy through metabolism, biochemical processes give rise to the complexity of life. Much of biochemistry deals with the structures, functions and interactions of cellular components such as proteins, carbohydrates, lipids, nucleic acids and other biomolecules —although increasingly processes rather than individual molecules are the main focus. Among the vast number of different biomolecules, many are complex and large molecules (called biopolymers), which are composed of similar repeating subunits (called monomers). Each class of polymeric biomolecule has a different set of subunit types.1 For example, a protein is a polymer whose subunits are selected from a set of 20 or more amino acids. Biochemistry studies the chemical properties of important biological molecules, like proteins, and in particular the chemistry of enzyme-catalyzed reactions. The biochemistry of cell metabolism and the endocrine system has been extensively described. Other areas of biochemistry include the genetic code (DNA, RNA), protein synthesis, cell membrane transport and signal transduction. Over the last 40 years biochemistry has become so successful at explaining living processes that now almost all areas of the life sciences from botany to medicine are engaged in biochemical research. Today the main focus of pure biochemistry is in understanding how biological molecules give rise to the processes that occur within living cells, which in turn relates greatly to the study and understanding of whole organisms. 2.Protein structure The particular series of amino acids that form a protein is known as that protein's primary structure. This sequence is determined by the genetic makeup of the individual. It specifies the order of side-chain groups along the linear polypeptide "backbone". Proteins have two types of well-classified, frequently occurring elements of local structure defined by a particular pattern of hydrogen bonds along the backbone: alpha helix and beta sheet. Their number and arrangement is called the secondary structure of the protein. Alpha helices are regular spirals stabilized by hydrogen bonds between the backbone CO group (carbonyl) of one amino acid residue and the backbone NH group (amide) of the i+4 residue. The spiral has about 3.6 amino acids per turn, and the amino acid side chains stick out from the cylinder of the helix. Beta pleated sheets are formed by backbone hydrogen bonds between individual beta strands each of which is in an "extended", or fully stretched-out, conformation. The strands may lie parallel or antiparallel to each other, and the side-chain direction alternates above and below the sheet. Hemoglobin contains only helices, natural silk is formed of beta pleated sheets, and many enzymes have a pattern of alternating helices and beta-strands. The secondary-structure elements are connected by "loop" or "coil" regions of non-repetitive conformation, which are sometimes quite mobile or disordered but usually adopt a well-defined, stable arrangement.8 The overall, compact, 3D structure of a protein is termed its tertiary structure or its "fold". It is formed as result of various attractive forces like hydrogen bonding, disulfide bridges, hydrophobic interactions, hydrophilic interactions, van der Waals force etc. When two or more polypeptide chains (either of identical or of different sequence) cluster to form a protein, quaternary structure of protein is formed. Quaternary structure is an attribute of polymeric (same-sequence chains) or heteromeric (different-sequence chains) proteins like hemoglobin, which consists of two "alpha" and two "beta" polypeptide chains. Apoenzymes An apoenzyme (or, generally, an apoprotein) is the protein without any smallmolecule cofactors, substrates, or inhibitors bound. It is often important as an inactive storage, transport, or secretory form of a protein. This is required, for instance, to protect the secretory cell from the activity of that protein. Apoenzymes becomes active enzymes on addition of a cofactor. Cofactors can be either inorganic (e.g., metal ions and iron-sulfur clusters) or organic compounds, (e.g., flavin and heme). Organic cofactors can be either prosthetic groups, which are tightly bound to an enzyme, or coenzymes, which are released from the enzyme's active site during the reaction. Isoenzymes Isoenzymes, or isozymes, are multiple forms of an enzyme, with slightly different protein sequence and closely similar but usually not identical functions. They are either products of different genes, or else different products of alternative splicing. They may either be produced in different organs or cell types to perform the same function, or several isoenzymes may be produced in the same cell type under diiferential regulation to suit the needs of changing development or environment. LDH (lactate dehydrogenase) has multiple isozymes, while fetal hemoglobin is an example of a developmentally regulated isoform of a nonenzymatic protein. The relative levels of isoenzymes in blood can be used to diagnose problems in the organ of secretion. Vitamins A vitamin is a compound that is generally not synthesized by a given organism but is nonetheless vital to its survival or health (for example coenzymes). These compounds must be absorbed, or eaten, but typically only in trace quantities. When originally proposed by Casimir Funk, a Polish biochemist, he believed them to all be basic and therefore named them vital amines. The "al" was later dropped to form the word vitamines. 3.History Gerty Cori and Carl Cori jointly won the Nobel Prize in 1947 for their discovery of the Cori cycle at RPMI. It once was generally believed that life and its materials had some essential property or substance distinct from any found in non-living matter, and it was thought that only living beings could produce the molecules of life. Then, in 1828, Friedrich Wöhler published a paper on the synthesis of urea, proving that organic compounds can be created artificially. The dawn of biochemistry may have been the discovery of the first enzyme, diastase (today called amylase), in 1833 by Anselme Payen. Eduard Buchner contributed the first demonstration of a complex biochemical process outside of a cell in 1896: alcoholic fermentation in cell extracts of yeast. Although the term “biochemistry” seems to have been first used in 1882, it is generally accepted that the formal coinage of biochemistry occurred in 1903 by Carl Neuberg, a German chemist. Previous to this time, this area would have been referred to as physiological chemistry Since then, biochemistry has advanced, especially since the mid-20th century, with the development of new techniques such as chromatography, X-ray diffraction, dual polarisation interferometry, NMR spectroscopy, radioisotopic labeling, electron microscopy, and molecular dynamics simulations. These techniques allowed for the discovery and detailed analysis of many molecules and metabolic pathways of the cell, such as glycolysis and the Krebs cycle (citric acid cycle). Another significant historic event in biochemistry is the discovery of the gene and its role in the transfer of information in the cell. This part of biochemistry is often called molecular biology. In the 1950s, James D. Watson, Francis Crick, Rosalind Franklin, and Maurice Wilkins were instrumental in solving DNA structure and suggesting its relationship with genetic transfer of information. In 1958, George Beadle and Edward Tatum received the Nobel Prize for work in fungi showing that one gene produces one enzyme. In 1988, Colin Pitchfork was the first person convicted of murder with DNA evidence, which led to growth of forensic science. More recently, Andrew Z. Fire and Craig C. Mello received the 2006 Nobel Prize for discovering the role of RNA interference (RNAi), in the silencing of gene expression. Starting materials: the chemical elements of life Around two dozen of the 94 naturally-occurring chemical elements are essential to various kinds of biological life. Most rare elements on Earth are not needed by life (exceptions being selenium and iodine), while a few common ones (aluminum and titanium) are not used. Most organisms share element needs, but there are a few differences between plants and animals. For example ocean algae use bromine but land plants and animals seem to need none. All animals require sodium, but some plants do not. Plants need boron and silicon, but animals may not (or may need ultra-small amounts). Just six elements—carbon, hydrogen, nitrogen, oxygen, calcium, and phosphorus—make up almost 99% of the mass of a human body (see composition of the human body for a complete list). In addition to the six major elements that compose most of the human body, humans require smaller amounts of possibly 18 more. 20 marks 1.Biomolecules The four main classes of molecules in biochemistry are carbohydrates, lipids, proteins, and nucleic acids. Many biological molecules are polymers: in this terminology, monomers are relatively small micromolecules that are linked together to create large macromolecules, which are known as polymers. When monomers are linked together to synthesize a biological polymer, they undergo a process called dehydration synthesis. Different macromolecules can assemble in larger complexes, often needed for biological activity. Carbohydrates A molecule of sucrose (glucose + fructose), a disaccharide. Carbohydrates are made from monomers called monosaccharides. Some of these monosaccharides include glucose (C6H12O6), fructose (C6H12O6), and deoxyribose (C5H10O4). When two monosaccharides undergo dehydration synthesis, water is produced, as two hydrogen atoms and one oxygen atom are lost from the two monosaccharides' hydroxyl group. Lipids A triglyceride with a glycerol molecule on the left and three fatty acids coming off it. Lipids are usually made from one molecule of glycerol combined with other molecules. In triglycerides, the main group of bulk lipids, there is one molecule of glycerol and three fatty acids. Fatty acids are considered the monomer in that case, and may be saturated (no double bonds in the carbon chain) or unsaturated (one or more double bonds in the carbon chain). Lipids, especially phospholipids, are also used in various pharmaceutical products, either as co-solubilisers (e.g., in parenteral infusions) or else as drug carrier components (e.g., in a liposome or transfersome). There are four ways in which a racemate can crystallize, three of which H. W. B. Roozeboom had distinguished by 1899: Conglomerate (sometimes racemic mixture or racemic conglomerate) A mechanical mixture of enantiomerically pure crystals of one enantiomer and its opposite. Molecules in the crystal structure have a greater affinity for the same enantiomer than for the opposite enantiomer. The melting point of the racemic conglomerate is always lower than that of the pure enantiomer. Addition of a small amount of one enantiomer to the conglomerate increases the melting point. Racemic compound (sometimes true racemate) Molecules have a greater affinity for the opposite enantiomer than for the same enantiomer; the substance forms a single crystalline phase in which the two enantiomers are present in an ordered 1:1 ratio in the elementary cell. Adding a small amount of one enantiomer to the racemic compound decreases the melting point. But the pure enantiomer can have a higher or lower melting point than the compound. Pseudoracemate (sometimes racemic solid solution) In contrast to the racemic compound or conglomerate, there is no big difference in affinity between the same and opposite enantiomers. Overall, both enantiomers occur in equal proportions in the crystal, but they coexist in an unordered manner in the crystal lattice. Addition of a small amount of one enantiomer changes the melting point just little bit or not at all. Quasiracemate A quasiracemate is a mixture of two similar but distinct compounds, one of which is left-handed and the other right-handed. Although chemically different, they are sterically similar (isosteric) and are still able to form a racemic crystalline phase. One of the first such racemates studied, by Pasteur in 1853, forms from a 1:2 mixture of the bis ammonium salt of (+)tartaric acid and the bis ammonium salt of (−)-malic acid in water. Reinvestigated in 2008,3 the crystals formed are dumbbell-shape with the central part consisting of ammonium (+)-bitartrate, whereas the outer parts are a quasiracemic mixture of ammonium (+)-bitartrate and ammonium (−)bimalate. Proteins The general structure of an α-amino acid, with the amino group on the left and the carboxyl group on the right. Proteins are very large molecules – macro-biopolymers – made from monomers called amino acids. There are 20 standard amino acids, each containing a carboxyl group, an amino group, and a side-chain (known as an "R" group). The "R" group is what makes each amino acid different, and the properties of the side-chains greatly influence the overall three-dimensional conformation of a protein. When amino acids combine, they form a special bond called a peptide bond through dehydration synthesis, and become a polypeptide, or protein. In order to determine whether two proteins are related, or in other words to decide whether they are homologous or not, scientists use sequence-comparison methods. Methods like Sequence Alignments and Structural Alignments are powerful tools that help scientists identify homologies between related molecules. The relevance of finding homologies among proteins goes beyond forming an evolutionary pattern of protein families. By finding how similar two protein sequences are, we acquire knowledge about their structure and therefore their function. Nucleic acids The structure of deoxyribonucleic acid (DNA), the picture shows the monomers being put together. Nucleic acids are the molecules that make up DNA, an extremely important substance that all cellular organisms use to store their genetic information. The most common nucleic acids are deoxyribonucleic acid and ribonucleic acid. Their monomers are called nucleotides. The most common nucleotides are adenine, cytosine, guanine, thymine, and uracil. Adenine binds with thymine and uracil; Thymine binds only with adenine; and cytosine and guanine can bind only with each other. Nucleotide structure Ribose structure indicating numbering of carbon atoms A nucleotide is composed of a nucleobase (nitrogenous base), a five-carbon sugar (either ribose or 2-deoxyribose), and one phosphate group.2 Without the phosphate group, the nucleobase and sugar compose a nucleoside. A nucleotide can thus also be called a nucleoside monophosphate. The phosphate groups form bonds with either the 2, 3, or 5-carbon of the sugar, with the 5-carbon site most common. Cyclic nucleotides form when the phosphate group is bound to two of the sugar's hydroxyl groups.1 Nucleotides contain either a purine or a pyrimidine base. Ribonucleotides are nucleotides in which the sugar is ribose. Deoxyribonucleotides are nucleotides in which the sugar is deoxyribose. Nucleic acids are polymeric macromolecules made from nucleotide monomers. In DNA, the purine bases are adenine and guanine, while the pyrimidines are thymine and cytosine. RNA uses uracil in place of thymine. Adenine always pairs with thymine by 2 hydrogen bonds, while guanine pairs with cytosine through 3 hydrogen bonds, each due to their unique structures. Synthesis Nucleotides can be synthesized by a variety of means both in vitro and in vivo. In vivo, nucleotides can be synthesized de novo or recycled through salvage pathways.3 The components used in de novo nucleotide synthesis are derived from biosynthetic precursors of carbohydrate and amino acid metabolism, and from ammonia and carbon dioxide. The liver is the major organ of de novo synthesis of all four nucleotides. De novo synthesis of pyrimidines and purines follows two different pathways. Pyrimidines are synthesized first from aspartate and carbamoyl-phosphate in the cytoplasm to the common precursor ring structure orotic acid, onto which a phosphorylated ribosyl unit is covalently linked. Purines, however, are first synthesized from the sugar template onto which the ring synthesis occurs. For reference, the syntheses of the purine and pyrimidine nucleotides are carried out by several enzymes in the cytoplasm of the cell, not within a specific organelle. Nucleotides undergo breakdown such that useful parts can be reused in synthesis reactions to create new nucleotides. In vitro, protecting groups may be used during laboratory production of nucleotides. A purified nucleoside is protected to create a phosphoramidite, which can then be used to obtain analogues not found in nature and/or to synthesize an oligonucleotide. 2.Carbohydrates The function of carbohydrates includes energy storage and providing structure. Sugars are carbohydrates, but not all carbohydrates are sugars. There are more carbohydrates on Earth than any other known type of biomolecule; they are used to store energy and genetic information, as well as play important roles in cell to cell interactions and communications. Monosaccharides Glucose The simplest type of carbohydrate is a monosaccharide, which among other properties contains carbon, hydrogen, and oxygen, mostly in a ratio of 1:2:1 (generalized formula CnH2nOn, where n is at least 3). Glucose, one of the most important carbohydrates, is an example of a monosaccharide. So is fructose, the sugar commonly associated with the sweet taste of fruits.5a Some carbohydrates (especially after condensation to oligo- and polysaccharides) contain less carbon relative to H and O, which still are present in 2:1 (H:O) ratio. Monosaccharides can be grouped into aldoses (having an aldehyde group at the end of the chain, e.g. glucose) and ketoses (having a keto group in their chain; e.g. fructose). Both aldoses and ketoses occur in an equilibrium (starting with chain lengths of C4) cyclic forms. These are generated by bond formation between one of the hydroxyl groups of the sugar chain with the carbon of the aldehyde or keto group to form a hemiacetal bond. This leads to saturated five-membered (in furanoses) or sixmembered (in pyranoses) heterocyclic rings containing one O as heteroatom. Disaccharides Sucrose: ordinary table sugar and probably the most familiar carbohydrate. Two monosaccharides can be joined together using dehydration synthesis, in which a hydrogen atom is removed from the end of one molecule and a hydroxyl group (—OH) is removed from the other; the remaining residues are then attached at the sites from which the atoms were removed. The H—OH or H2O is then released as a molecule of water, hence the term dehydration. The new molecule, consisting of two monosaccharides, is called a disaccharide and is conjoined together by a glycosidic or ether bond. The reverse reaction can also occur, using a molecule of water to split up a disaccharide and break the glycosidic bond; this is termed hydrolysis. The most well-known disaccharide is sucrose, ordinary sugar (in scientific contexts, called table sugar or cane sugar to differentiate it from other sugars). Sucrose consists of a glucose molecule and a fructose molecule joined together. Another important disaccharide is lactose, consisting of a glucose molecule and a galactose molecule. As most humans age, the production of lactase, the enzyme that hydrolyzes lactose back into glucose and galactose, typically decreases. This results in lactase deficiency, also called lactose intolerance. Sugar polymers are characterised by having reducing or non-reducing ends. A reducing end of a carbohydrate is a carbon atom that can be in equilibrium with the open-chain aldehyde or keto form. If the joining of monomers takes place at such a carbon atom, the free hydroxy group of the pyranose or furanose form is exchanged with an OH-side-chain of another sugar, yielding a full acetal. This prevents opening of the chain to the aldehyde or keto form and renders the modified residue non-reducing. Lactose contains a reducing end at its glucose moiety, whereas the galactose moiety form a full acetal with the C4-OH group of glucose. Saccharose does not have a reducing end because of full acetal formation between the aldehyde carbon of glucose (C1) and the keto carbon of fructose (C2). Oligosaccharides and polysaccharides Cellulose as polymer of β-D-glucose When a few (around three to six) monosaccharides are joined together, it is called an oligosaccharide (oligo- meaning "few"). These molecules tend to be used as markers and signals, as well as having some other uses. Many monosaccharides joined together make a polysaccharide. They can be joined together in one long linear chain, or they may be branched. Two of the most common polysaccharides are cellulose and glycogen, both consisting of repeating glucose monomers. Cellulose is made by plants and is an important structural component of their cell walls. Humans can neither manufacture nor digest it. Glycogen, on the other hand, is an animal carbohydrate; humans and other animals use it as a form of energy storage. Use of carbohydrates as an energy source Glucose is the major energy source in most life forms. For instance, polysaccharides are broken down into their monomers (glycogen phosphorylase removes glucose residues from glycogen). Disaccharides like lactose or sucrose are cleaved into their two component monosaccharides. Glycolysis (anaerobic) Glucose is mainly metabolized by a very important ten-step pathway called glycolysis, the net result of which is to break down one molecule of glucose into two molecules of pyruvate; this also produces a net two molecules of ATP, the energy currency of cells, along with two reducing equivalents in the form of converting NAD+ to NADH. This does not require oxygen; if no oxygen is available (or the cell cannot use oxygen), the NAD is restored by converting the pyruvate to lactate (lactic acid) (e.g., in humans) or to ethanol plus carbon dioxide (e.g., in yeast). Other monosaccharides like galactose and fructose can be converted into intermediates of the glycolytic pathway. Aerobic In aerobic cells with sufficient oxygen, as in most human cells, the pyruvate is further metabolized. It is irreversibly converted to acetyl-CoA, giving off one carbon atom as the waste product carbon dioxide, generating another reducing equivalent as NADH. The two molecules acetyl-CoA (from one molecule of glucose) then enter the citric acid cycle, producing two more molecules of ATP, six more NADH molecules and two reduced (ubi)quinones (via FADH2 as enzymebound cofactor), and releasing the remaining carbon atoms as carbon dioxide. The produced NADH and quinol molecules then feed into the enzyme complexes of the respiratory chain, an electron transport system transferring the electrons ultimately to oxygen and conserving the released energy in the form of a proton gradient over a membrane (inner mitochondrial membrane in eukaryotes). Thus, oxygen is reduced to water and the original electron acceptors NAD+ and quinone are regenerated. This is why humans breathe in oxygen and breathe out carbon dioxide. The energy released from transferring the electrons from high-energy states in NADH and quinol is conserved first as proton gradient and converted to ATP via ATP synthase. This generates an additional 28 molecules of ATP (24 from the 8 NADH + 4 from the 2 quinols), totaling to 32 molecules of ATP conserved per degraded glucose (two from glycolysis + two from the citrate cycle). It is clear that using oxygen to completely oxidize glucose provides an organism with far more energy than any oxygen-independent metabolic feature, and this is thought to be the reason why complex life appeared only after Earth's atmosphere accumulated large amounts of oxygen. Gluconeogenesis In vertebrates, vigorously contracting skeletal muscles (during weightlifting or sprinting, for example) do not receive enough oxygen to meet the energy demand, and so they shift to anaerobic metabolism, converting glucose to lactate. The liver regenerates the glucose, using a process called gluconeogenesis. This process is not quite the opposite of glycolysis, and actually requires three times the amount of energy gained from glycolysis (six molecules of ATP are used, compared to the two gained in glycolysis). Analogous to the above reactions, the glucose produced can then undergo glycolysis in tissues that need energy, be stored as glycogen (or starch in plants), or be converted to other monosaccharides or joined into di- or oligosaccharides. The combined pathways of glycolysis during exercise, lactate's crossing via the bloodstream to the liver, subsequent gluconeogenesis and release of glucose into the bloodstream is called the Cori cycle. 3.Structure and nomenclature With few exceptions (e.g., deoxyribose), monosaccharides have the chemical formula Cx(H2O)y, where x is at least 3. Monosaccharides can be classified by the number x of carbon atoms they contain: diose (2) triose (3) tetrose (4), pentose (5), hexose (6), heptose (7), and so on. The most important monosaccharide, glucose, is a hexose. Examples of heptoses include the ketoses mannoheptulose and sedoheptulose. Monosaccharides with eight or more carbons are rarely observed as they are quite unstable. Linear-chain monosaccharides Simple monosaccharides have a linear and unbranched carbon skeleton with one carbonyl (C=O) functional group, and one hydroxyl (OH) group on each of the remaining carbon atoms. Therefore, the molecular structure of a simple monosaccharide can be written as H(CHOH)n(C=O)(CHOH)mH, where n+1+m = x; so that its elemental formula is CxH2xOx. By convention, the carbon atoms are numbered from 1 to x along the backbone, starting from the end that is closest to the C=O group. If the carbonyl is at position 1 (that is, n or m is zero), the molecule begins with an formyl group H(C=O)-, and is technically an aldehyde. In that case, the compound is termed an aldose. Otherwise, the molecule has a keto group, a carbonyl -(C=O)between two carbons; then it is formally a ketone, and is termed a ketose. Ketoses of biological interest usually have the carbonyl at position 2. The various classifications above can be combined, resulting in names like "aldohexose" and "ketotriose". A more general nomenclature for open chain monosaccharides combines a Greek prefix to indicate the number of carbons (tri-, tetr-, pent-, hex-, etc.), with the suffixes '-ose' for aldoses and '-ulose' for ketoses. In the latter case, if the carbonyl is not at position 2, its position is indicated by a numeric infix. So, for example, H(C=O)(CHOH)4H is pentose, H(CHOH)(C=O)(CHOH)3H is pentulose, and H(CHOH)2(C=O)(CHOH)2H is pent-3-ulose. Open-chain stereoisomers Two monosaccharides with equivalent molecular graphs (same chain length and same carbonyl position) may still be distinct stereoisomers, whose molecules differ in the three-dimensional arrangement of the bonds of certain atoms. This happens only if the molecule contains a stereogenic center, specifically a carbon atom that is chiral (connected to four distinct molecular sub-structures). Those four bonds can have any of two configurations in space distinguished by their handedness. In a simple open-chain monosaccharide, every carbon is chiral except the first and the last atoms of the chain, and (in ketoses) the carbon with the keto group. For example, the triketose H(CHOH)(C=O)(CHOH)H (glycerone, dihydroxyacetone) has no stereogenic center, and therefore exists as a single stereoisomer. The other triose, the aldose H(C=O)(CHOH)2H (glyceraldehyde), has one chiral carbon — the central one, number 2 — which is bonded to groups -H, -OH, -C(OH)H2, and -(C=O)H. Therefore, it exists as two stereoisomers whose molecules are mirror images of each other (like a left and a right glove). Monosaccharides with four or more carbons may contain multiple chiral carbons, so they typically have more than two stereoisomers. The number of distinct stereoisomers with the same diagram is bounded by 2c, where c is the number of chiral carbons. The Fischer projection is a systematic way of drawing the skeletal formula of an acyclic monosaccharide so that the handedness of each chiral carbon is well specified. Each stereoisomer of a simple open-chain monosaccharide can be identified by the positions (right or left) in the Fischer diagram of the chiral hydroxyls (the hydroxyls attached to the chiral carbons). Most stereoisomers are themselves chiral (distinct from their mirror images). In the Fischer projection, two mirror-image isomers differ by having the positions of all chiral hydroxyls reversed right-to-left. Mirror-image isomers are chemically identical in non-chiral environments, but usually have very different biochemical properties and occurrences in nature. While most stereoisomers can be arranged in pairs of mirror-image forms, there are some non-chiral stereoisomers that are identical to their mirror images, in spite of having chiral centers. This happens whenever the molecular graph is symmetrical, as in the 3-ketopentoses H(CHOH)2(CO)(CHOH)2H, and the two halves are mirror images of each other. In that case, mirroring is equivalent to a half-turn rotation. For this reason, there are only three distinct 3-ketopentose stereoisomers, even though the molecule has two chiral carbons. Distinct stereoisomers that are not mirror-images of each other usually have different chamical properties, even in non-chiral environments. Therefore, each mirror pair and each non-chiral stereoisomer may be given a specific monosaccharide name. For example, there are 16 distinct aldohexose stereoisomers, but the name "glucose" means a specific pair of mirror-image aldohexoses. In the Fischer projection, one of the two glucose isomers has the hydroxyl at left on C3, and at right on C4 and C5; while the other isomer has the reversed pattern. These specific monosaccharide names have conventional threeletter abbreviations, like 'Glu' for glucose and 'Thr' for threose. Generally, a monosaccharide with n asymmetrical carbons has 2n stereoisomers. The number of open chain stereoisomers for an aldose monosaccharide is larger by one than that of a ketose monosaccharide of the same length. Every ketose will have 2(n-3) stereoisomers where n > 2 is the number of carbons. Every aldose will have 2(n-2) stereoisomers where n > 2 is the number of carbons. 4. MOLECULAR DYNAMICS Molecular dynamics (MD) is a computer simulation of physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a period of time, giving a view of the motion of the atoms. In the most common version, the trajectories of molecules and atoms are determined by numerically solving the Newton's equations of motion for a system of interacting particles, where forces between the particles and potential energy are defined by molecular mechanics force fields. The method was originally conceived within theoretical physics in the late 1950s1 and early 1960s ,2 but is applied today mostly in materials science and the modeling of biomolecules. Because molecular systems consist of a vast number of particles, it is impossible to find the properties of such complex systems analytically; MD simulation circumvents this problem by using numerical methods. However, long MD simulations are mathematically ill-conditioned, generating cumulative errors in numerical integration that can be minimized with proper selection of algorithms and parameters, but not eliminated entirely. The results of molecular dynamics simulations may be used to determine macroscopic thermodynamic properties of the system based on the ergodic hypothesis: the statistical ensemble averages are equal to time averages of the system. MD has also been termed "statistical mechanics by numbers" and "Laplace's vision of Newtonian mechanics" of predicting the future by animating nature's forces34 and allowing insight into molecular motion on an atomic scale. Example of a molecular dynamics simulation in a simple system: deposition of a single Cu atom on a Cu (001) surface. Each circle illustrates the position of a single atom; note that the actual atomic interactions used in current simulations are more complex than those of 2-dimensional hard spheres. Highly simplified description of the molecular dynamics simulation algorithm. The simulation proceeds iteratively by alternatively calculating forces and solving the equations of motion based on the accelerations obtained from the new forces. In practise, almost all MD codes use much more complicated versions of the algorithm, including two steps (predictor and corrector) in solving the equations of motion and many additional steps for e.g. temperature and pressure control, analysis and output. History Before it became possible to simulate molecular dynamics with computers, some undertook the hard work of trying it with physical models such as macroscopic spheres. The idea was to arrange them to replicate the properties of a liquid. J.D. Bernal said, in 1962: "... I took a number of rubber balls and stuck them together with rods of a selection of different lengths ranging from 2.75 to 4 inches. I tried to do this in the first place as casually as possible, working in my own office, being interrupted every five minutes or so and not remembering what I had done before the interruption."5 Fortunately, now computers keep track of bonds during a simulation. Areas of Application There is a significant difference between the focus and methods used by chemists and physicists, and this is reflected in differences in the jargon used by the different fields. In chemistry and biophysics, the interaction between the particles is either described by a "force field" (classical MD), a quantum chemical model, or a mix between the two. These terms are not used in physics, where the interactions are usually described by the name of the theory or approximation being used and called the potential energy, or just the "potential".