* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Making Transgenic Plants and Animals
Zinc finger nuclease wikipedia , lookup
Point mutation wikipedia , lookup
X-inactivation wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Genome (book) wikipedia , lookup
Epigenetics in stem-cell differentiation wikipedia , lookup
Gene therapy wikipedia , lookup
Mir-92 microRNA precursor family wikipedia , lookup
Helitron (biology) wikipedia , lookup
Genetic engineering wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Microevolution wikipedia , lookup
Designer baby wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
History of genetic engineering wikipedia , lookup
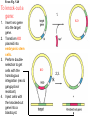
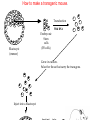
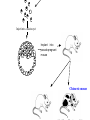
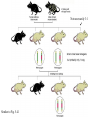
Making Transgenic Plants and Animals • Why? 1. Study gene function and regulation 2. Generate new organismic tools for other fields of research. 3. Cure genetic diseases. 4. Improve agriculture and related raw materials. 5. Generate new systems or sources for bioengineered drugs (e.g., use plants instead of animals or bacteria). The organism of choice for mammalian genetic engineers. - small - hardy - short life cycle - genetics possible - many useful strains and tools The Nobel Prize in Physiology or Medicine, 2007 Mario R. Capecchi, Martin J. Evans and Oliver Smithies for their discoveries of "principles for introducing specific gene modifications in mice by the use of embryonic stem cells" M. Capecchi Univ. of Utah Sir M. Evans Cardiff Univ., UK O. Smithies UNC Chapel Hill The Problem with trying to make KOs: Random DNA Integration • DNA can integrate into the genome by homologous (H) or non-homologous (N-H) recombination • Frequency of N-H >> H (by at least 5000-fold) in mammalian cells • If you want H integrants, which you need for knock-outs, you must have a selection scheme for those. Vector for integrating a transgene by HR (i.e., into a specific site) tk1 & tk2 - two copies of a Herpes Simplex Virus thymidine kinase gene (makes cells susceptible to gancyclovir) Neo - neomycin resistance gene Homologous regions - homologous to the chromosomal target Transgene - foreign gene Example of what happens with N-H recombination Nonhomologous recombination homologous tk1 sequence neo transgene homologous sequence tk2 chromosome tk1 homologous sequence neo transgene homologous sequence tk2 chromosome Transformed cells are neo-resistant, but gancyclovir sensitive. homol--> What happens with HR Homologous recombinants homologous tk1 sequence neo transgene homologous sequence tk2 chromosome homologous sequence neo transgene homologous sequence chromosome If DNA goes in by HR, transformed cells are both neo-resistant and gancyclovir-resistant! Use double-selection to get only those cells with a homologous integration event. From Fig. 5.40 To knock-out a gene: 1. Insert neo gene into the target gene. 2. Transform KO plasmid into embryonic stem cells. 3. Perform doubleselection to get cells with the homologous integration (neo & gangcyclovir resistant). 4. Inject cells with the knocked-out gene into a blastocyst. 1. KO KO 2,3. How to make a transgenic mouse. Transfection With DNA Blastocyst Embryonic Stem cells (ES cells) (mouse) Grow in culture. Select for those that carry the transgene. Inject into a blastocyst Inject into a blastocyst Implant int o pseudo pregn an t mou se Chimeric mouse (a) If the recipient ES cells are from a brown mouse, and the transformed (transgenic) ES cells are injected into a black (female) mouse, chimeras are easily identified by their Brown/Black phenotype. (b) To obtain a completely transgenic KO mouse (where all cells have a KO gene), mate the chimera with a black mouse. Some of the progeny will be brown (which is dominant), indicating fertilization with a germ-line cell (gamete) that ultimately came from a KO-ES cell. Only about 50% of the brown progeny mice, however, will have the KO allele, because the transgenic ES cell that underwent meiosis to produce the germ-line cell was probably heterozygous for the KOed gene. (c) To obtain a homozygous KO mouse (both alleles are KOs), cross brown heterozygotes, and ~1/4 of the progeny will be homozygous. Not necessarily 3:1 Similar to Fig. 5.41 Gene therapy in humans presents some formidable problems • If you could introduce the gene in early development (e.g., eggs? or blastocyst) might could cure (or partially cure) many diseases. • How to fix them later, as a child, adolescent, adult, etc.? • Transgenic technology + stem cell technology = many interesting possibilities