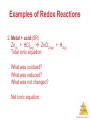* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 4 - AP Chemistry with dr hart
Cracking (chemistry) wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Nuclear fusion wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Double layer forces wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Organic chemistry wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Asymmetric induction wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Rate equation wikipedia , lookup
Electrolysis of water wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Hydroformylation wikipedia , lookup
Acid strength wikipedia , lookup
Marcus theory wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Stoichiometry wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Transition state theory wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Click chemistry wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Liquid–liquid extraction wikipedia , lookup
Ene reaction wikipedia , lookup
Electrochemistry wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Acid–base reaction wikipedia , lookup
Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 4 Aqueous Reactions and Solution Stoichiometry John D. Bookstaver St. Charles Community College Cottleville, MO Aqueous Reactions © 2009, Prentice-Hall, Inc. Solutions • Solutions are defined as homogeneous mixtures of two or more pure substances. • The solvent is present in greatest abundance. • All other substances are solutes. Aqueous Reactions © 2009, Prentice-Hall, Inc. Dissociation • When an ionic substance dissolves in water, the solvent pulls the individual ions from the crystal and solvates them. • This process is called dissociation. Aqueous Reactions © 2009, Prentice-Hall, Inc. Dissociation • An electrolyte is a substances that dissociates into ions when dissolved in water. Aqueous Reactions © 2009, Prentice-Hall, Inc. Electrolytes • An electrolyte is a substances that dissociates into ions when dissolved in water. • A nonelectrolyte may dissolve in water, but it does not dissociate into ions when it does Aqueous so. Reactions © 2009, Prentice-Hall, Inc. Electrolytes and Nonelectrolytes Soluble ionic compounds tend to be electrolytes. Aqueous Reactions © 2009, Prentice-Hall, Inc. Electrolytes and Nonelectrolytes Molecular compounds tend to be nonelectrolytes, except for acids and bases. Aqueous Reactions © 2009, Prentice-Hall, Inc. Electrolytes • A strong electrolyte dissociates completely when dissolved in water. • A weak electrolyte only dissociates partially when dissolved in water. Aqueous Reactions © 2009, Prentice-Hall, Inc. Strong Electrolytes Are… • Strong acids • Strong bases Aqueous Reactions © 2009, Prentice-Hall, Inc. Strong Electrolytes Are… • Strong acids • Strong bases • Soluble ionic salts Aqueous Reactions © 2009, Prentice-Hall, Inc. Sample Exercise 4.1 (p. 127) The diagram below represents an aqueous solution of one of the following compounds: MgCl2, KCl, or K2SO4. Which solution does it best represent? Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.1) If you have an aqueous solution that contains 1.5 moles of HCl, how many moles of ions are in the solution? a) 1.0 b) 1.5 c) 2.0 d) 2.5 e) 3.0 Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.1) If you have an aqueous solution that contains 1.5 moles of HCl, how many moles of ions are in the solution? a) 1.0 b) 1.5 c) 2.0 d) 2.5 e) 3.0 Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 2 (4.1) If you were to draw diagrams (such as that shown on the left of p. 128) representing aqueous solutions of each of the following ionic compounds, how many anions would you show if the diagram contained six cations? a)NiSO4 b)Ca(NO3)2 c) Na3PO4 d)Al2(SO4)3 Aqueous Reactions © 2009, Prentice-Hall, Inc. Precipitation Reactions When one mixes ions that form compounds that are insoluble (as could be predicted by the solubility guidelines), a precipitate is formed. Aqueous Reactions © 2009, Prentice-Hall, Inc. Aqueous Reactions © 2009, Prentice-Hall, Inc. Sample Exercise 4.2 (p. 130) Classify the following ionic compounds as soluble or insoluble in water: a) sodium carbonate (Na2CO3) b) lead sulfate (PbSO4) Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.2) Which of the following compounds is insoluble in water? a) (NH4)2S b) CaCO3 c) NaOH d) Ag2SO4 e) Pb(CH3COO)2 Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.2) Which of the following compounds is insoluble in water? a) (NH4)2S b) CaCO3 c) NaOH d) Ag2SO4 e) Pb(CH3COO)2 Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 2 (4.2) Classify the following compounds as soluble or insoluble in water: a) cobalt (II) hydroxide b) barium nitrate c) ammonium phosphate Aqueous Reactions © 2009, Prentice-Hall, Inc. Metathesis (Exchange) Reactions • Metathesis comes from a Greek word that means “to transpose.” AgNO3 (aq) + KCl (aq) AgCl (s) + KNO3 (aq) Aqueous Reactions © 2009, Prentice-Hall, Inc. Metathesis (Exchange) Reactions • Metathesis comes from a Greek word that means “to transpose.” • It appears the ions in the reactant compounds exchange, or transpose, ions. AgNO3 (aq) + KCl (aq) AgCl (s) + KNO3 (aq) Aqueous Reactions © 2009, Prentice-Hall, Inc. Sample Exercise 4.3 (p. 131) a) Predict the identity of the precipitate that forms when solutions of BaCl2 and K2SO4 are mixed. b) Write the balanced chemical equation for the reaction. Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.3) Yes or No: Will a precipitate form when solutions of Ba(NO3)2 and KOH are mixed? Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.3) Yes or No: Will a precipitate form when solutions of Ba(NO3)2 and KOH are mixed? Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 2 (4.3) a) What compound precipitates when solutions of Fe2(SO4)3 and LiOH are mixed? b) Write a balanced equation for the reaction. c) Will a precipitate form when solutions of Ba(NO3)2 and KOH are mixed? Aqueous Reactions © 2009, Prentice-Hall, Inc. Solution Chemistry • It is helpful to pay attention to exactly what species are present in a reaction mixture (i.e., solid, liquid, gas, aqueous solution). • If we are to understand reactivity, we must be aware of just what is changing during the course of a reaction. Aqueous Reactions © 2009, Prentice-Hall, Inc. Molecular Equation The molecular equation lists the reactants and products in their molecular form. AgNO3 (aq) + KCl (aq) AgCl (s) + KNO3 (aq) Aqueous Reactions © 2009, Prentice-Hall, Inc. Ionic Equation • In the ionic equation all strong electrolytes (strong acids, strong bases, and soluble ionic salts) are dissociated into their ions. • This more accurately reflects the species that are found in the reaction mixture. Ag+ (aq) + NO3- (aq) + K+ (aq) + Cl- (aq) AgCl (s) + K+ (aq) + NO3- (aq) Aqueous Reactions © 2009, Prentice-Hall, Inc. Net Ionic Equation • To form the net ionic equation, cross out anything that does not change from the left side of the equation to the right. Ag+(aq) + NO3-(aq) + K+(aq) + Cl-(aq) AgCl (s) + K+(aq) + NO3-(aq) Aqueous Reactions © 2009, Prentice-Hall, Inc. Net Ionic Equation • To form the net ionic equation, cross out anything that does not change from the left side of the equation to the right. • The only things left in the equation are those things that change (i.e., react) during the course of the reaction. Ag+(aq) + Cl-(aq) AgCl (s) Aqueous Reactions © 2009, Prentice-Hall, Inc. Net Ionic Equation • To form the net ionic equation, cross out anything that does not change from the left side of the equation to the right. • The only things left in the equation are those things that change (i.e., react) during the course of the reaction. • Those things that didn’t change (and were deleted from the net ionic equation) are called spectator ions. Ag+(aq) + NO3-(aq) + K+(aq) + Cl-(aq) AgCl (s) + K+(aq) + NO3-(aq) Aqueous Reactions © 2009, Prentice-Hall, Inc. Writing Net Ionic Equations 1. Write a balanced molecular equation. 2. Dissociate all strong electrolytes. 3. Cross out anything that remains unchanged from the left side to the right side of the equation. 4. Write the net ionic equation with the species that remain. Aqueous Reactions © 2009, Prentice-Hall, Inc. Sample Exercise 4.4 (p. 132) Write the a) molecular equation b) total ionic equation and the c) net ionic equation for the precipitation reaction that occurs when solutions of calcium chloride and sodium carbonate are mixed. Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.4) What happens when you mix an aqueous solution of sodium nitrate with an aqueous solution of barium chloride? a) There is no reaction; all possible products are soluble. b) Only barium nitrate precipitates. c) Only sodium chloride precipitates. d) Both barium nitrate and sodium chloride precipitate. e) Nothing; barium chloride is not soluble and itAqueous Reactions stays as a precipitate. © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.4) What happens when you mix an aqueous solution of sodium nitrate with an aqueous solution of barium chloride? a) There is no reaction; all possible products are soluble. b) Only barium nitrate precipitates. c) Only sodium chloride precipitates. d) Both barium nitrate and sodium chloride precipitate. e) Nothing; barium chloride is not soluble and itAqueous Reactions stays as a precipitate. © 2009, Prentice-Hall, Inc. Practice Exercise 2 (4.4) Write the net ionic equation for the precipitation reaction that occurs when aqueous solutions of silver nitrate and potassium phosphate are mixed. Aqueous Reactions © 2009, Prentice-Hall, Inc. Acids • Arrhenius defined acids as substances that increase the concentration of H+ when dissolved in water. • Brønsted and Lowry defined them as proton donors. Aqueous Reactions © 2009, Prentice-Hall, Inc. Acids There are only seven strong acids: • • • • • • • Hydrochloric (HCl) Hydrobromic (HBr) Hydroiodic (HI) Nitric (HNO3) Sulfuric (H2SO4) Chloric (HClO3) Perchloric (HClO4) Aqueous Reactions © 2009, Prentice-Hall, Inc. Bases • Arrhenius defined bases as substances that increase the concentration of OH− when dissolved in water. • Brønsted and Lowry defined them as proton acceptors. Aqueous Reactions © 2009, Prentice-Hall, Inc. Bases The strong bases are the soluble metal salts of hydroxide ion: • • • • Alkali metals Calcium Strontium Barium Aqueous Reactions © 2009, Prentice-Hall, Inc. Acid-Base Reactions In an acid-base reaction, the acid donates a proton (H+) to the base. Aqueous Reactions © 2009, Prentice-Hall, Inc. Sample Exercise 4.5 (p. 134) The diagrams below represent aqueous solutions of three acids (HX, HY, and HZ) with water molecules omitted for clarity. Rank them from strongest to weakest. Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.5) A set of aqueous solutions are prepared containing different acids at the same concentration: acetic acid, chloric acid and hydrobromic acid. Which solution(s) are the most electrically conductive? a) chloric acid b) hydrobromic acid c) acetic acid d) both chloric acid and hydrobromic acid e) all three solutions have the same electrical conductivity Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.5) A set of aqueous solutions are prepared containing different acids at the same concentration: acetic acid, chloric acid and hydrobromic acid. Which solution(s) are the most electrically conductive? a) chloric acid b) hydrobromic acid c) acetic acid d) both chloric acid and hydrobromic acid e) all three solutions have the same electrical conductivity Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 2 (4.5) Imagine a diagram showing 10 Na+ ions and 10 OH- ions. If this solution were mixed with the one pictured above for HY, what would the diagram look like that represents the solution after any possible reaction? Aqueous Reactions © 2009, Prentice-Hall, Inc. Sample Exercise 4.6 (p. 135) Classify each of the following dissolved substances as a strong electrolyte, weak electrolyte, or nonelectrolyte: CaCl2 HNO3 C2H5OH (ethanol) HCHO2 (formic acid) KOH Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.6) Which of these substances, when dissolved in water, is a strong electrolyte? a) ammonia b) hydrofluoric acid c) folic acid d) sodium nitrate e) sucrose Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.6) Which of these substances, when dissolved in water, is a strong electrolyte? a) ammonia b) hydrofluoric acid c) folic acid d) sodium nitrate e) sucrose Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 2 (4.6) Consider solutions in which 0.1 mol of each of the following compounds is dissolved in 1 L of water: Ca(NO3)2 (calcium nitrate), C6H12O6 (glucose), NaC2H3O2 (sodium acetate), and HC2H3O2 (acetic acid). Rank the solutions in order of increasing electrical conductivity, based on the fact that the greater the number of ions in solution, the greater the conductivity. Aqueous Reactions © 2009, Prentice-Hall, Inc. Neutralization Reactions Generally, when solutions of an acid and a base are combined, the products are a salt and water. CH3COOH (aq) + NaOH (aq) CH3COONa (aq) + H2O (l) Aqueous Reactions © 2009, Prentice-Hall, Inc. Neutralization Reactions When a strong acid reacts with a strong base, the net ionic equation is… HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l) Aqueous Reactions © 2009, Prentice-Hall, Inc. Neutralization Reactions When a strong acid reacts with a strong base, the net ionic equation is… HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l) H+ (aq) + Cl- (aq) + Na+ (aq) + OH-(aq) Na+ (aq) + Cl- (aq) + H2O (l) Aqueous Reactions © 2009, Prentice-Hall, Inc. Neutralization Reactions When a strong acid reacts with a strong base, the net ionic equation is… HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l) H+ (aq) + Cl- (aq) + Na+ (aq) + OH-(aq) Na+ (aq) + Cl- (aq) + H2O (l) H+ (aq) + OH- (aq) H2O (l) Aqueous Reactions © 2009, Prentice-Hall, Inc. Sample Exercise 4.7 (p. 136) a) Write a balanced complete chemical equation for the reaction between aqueous solutions of acetic acid (CH3COOH) and barium hydroxide (Ba(OH)2). b) Write the net ionic equation for this reaction. Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.7) Which is the correct net ionic equation for the reaction of aqueous ammonia with nitric acid? a) NH4+(aq) + H+(aq) NH52+(aq) b) NH3(aq) + NO3-(aq) NH2-(aq) + HNO3(aq) c) NH2-(aq) + H+(aq) NH3(aq) d) NH3(aq) + H+(aq) NH4+(aq) e) NH4+(aq) + NO3-(aq) NH4NO3(aq) Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.7) Which is the correct net ionic equation for the reaction of aqueous ammonia with nitric acid? a) NH4+(aq) + H+(aq) NH52+(aq) b) NH3(aq) + NO3-(aq) NH2-(aq) + HNO3(aq) c) NH2-(aq) + H+(aq) NH3(aq) d) NH3(aq) + H+(aq) NH4+(aq) e) NH4+(aq) + NO3-(aq) NH4NO3(aq) Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 2 (4.7) For the reaction of phosphorous acid (H3PO4) and potassium hydroxide (KOH), write a) the balanced molecular equation b) the net ionic equation Aqueous Reactions © 2009, Prentice-Hall, Inc. Gas-Forming Reactions • Some metathesis reactions do not give the product expected. • In this reaction, the expected product (H2CO3) decomposes to give a gaseous product (CO2). CaCO3 (s) + HCl (aq) CaCl2 (aq) + CO2 (g) + H2O (l) Aqueous Reactions © 2009, Prentice-Hall, Inc. Gas-Forming Reactions When a carbonate or bicarbonate reacts with an acid, the products are a salt, carbon dioxide, and water. CaCO3 (s) + HCl (aq) CaCl2 (aq) + CO2 (g) + H2O (l) NaHCO3 (aq) + HBr (aq) NaBr (aq) + CO2 (g) + H2O (l) Aqueous Reactions © 2009, Prentice-Hall, Inc. Gas-Forming Reactions Similarly, when a sulfite reacts with an acid, the products are a salt, sulfur dioxide, and water. SrSO3 (s) + 2 HI (aq) SrI2 (aq) + SO2 (g) + H2O (l) Aqueous Reactions © 2009, Prentice-Hall, Inc. Gas-Forming Reactions • This reaction gives the predicted product, but you had better carry it out in the hood, or you will be very unpopular! • But just as in the previous examples, a gas is formed as a product of this reaction. Na2S (aq) + H2SO4 (aq) Na2SO4 (aq) + H2S (g) Aqueous Reactions © 2009, Prentice-Hall, Inc. Oxidation-Reduction Reactions • An oxidation occurs when an atom or ion loses electrons. • A reduction occurs when an atom or ion gains electrons. • One cannot occur without the other. Aqueous Reactions © 2009, Prentice-Hall, Inc. Oxidation Numbers To determine if an oxidation-reduction reaction has occurred, we assign an oxidation number to each element in a neutral compound or charged entity. Aqueous Reactions © 2009, Prentice-Hall, Inc. Oxidation Numbers • Elements in their elemental form have an oxidation number of 0. • The oxidation number of a monatomic ion is the same as its charge. Aqueous Reactions © 2009, Prentice-Hall, Inc. Oxidation Numbers • Nonmetals tend to have negative oxidation numbers, although some are positive in certain compounds or ions. Oxygen has an oxidation number of −2, except in the peroxide ion in which it has an oxidation number of −1. Hydrogen is −1 when bonded to a metal, +1 when bonded to a nonmetal. Aqueous Reactions © 2009, Prentice-Hall, Inc. Oxidation Numbers • Nonmetals tend to have negative oxidation numbers, although some are positive in certain compounds or ions. Fluorine always has an oxidation number of −1. The other halogens have an oxidation number of −1 when they are negative; they can have positive oxidation numbers, Aqueous however, most notably in oxyanions. Reactions © 2009, Prentice-Hall, Inc. Oxidation Numbers • The sum of the oxidation numbers in a neutral compound is 0. • The sum of the oxidation numbers in a polyatomic ion is the charge on the ion. Aqueous Reactions © 2009, Prentice-Hall, Inc. Examples of Oxidation-Reduction Reactions Mg(s) + 2 HCl(aq) MgCl2(aq) + H2(g) 0 2 x+1 2x-1 +2 2x-1 0 Note: The transfer of electrons during oxidation-reduction reactions often produce energy (when spontaneous), which can be in the form of electricity. And electrical energy can be used to make nonspontaneous chemical reactions occur. Aqueous Reactions © 2009, Prentice-Hall, Inc. Examples of Redox Reactions 1.Combustion: “rapid oxidation reaction in which a large amount of heat and usually light are released” C + O2 CO2, CO S + O2 SO2 What was oxidized? What was reduced? Aqueous Reactions © 2009, Prentice-Hall, Inc. Examples of Redox Reactions 2. Metal + acid (SR) Zn(s) + HCl(aq) ZnCl2(aq) + H2(g) Total ionic equation: What was oxidized? What was reduced? What was not changed? Net Ionic equation: Aqueous Reactions © 2009, Prentice-Hall, Inc. Examples of Redox Reactions 3. Metal + salt (SR) Mg(s) + CoSO4(aq) MgSO4(aq) + Co(s) Total ionic equation: What was oxidized? What was reduced? Aqueous Reactions © 2009, Prentice-Hall, Inc. Sample Exercise 4.8 (p. 141) Determine the oxidation state of sulfur in each of the following: a) H2S b) S8 c) SCl2 d) Na2SO3 e) SO42Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.8) In which compound is the oxidation state of oxygen -1? a) O2 b) H2O c) H2SO4 d) H2O2 e) KCH3COO Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.8) In which compound is the oxidation state of oxygen -1? a) O2 b) H2O c) H2SO4 d) H2O2 e) KCH3COO Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 2 (4.8) What is the oxidation state of the boldfaced element in each of the following: a) P2O5 b) NaH c) Cr2O72d) SnBr4 e) BaO2 Aqueous Reactions © 2009, Prentice-Hall, Inc. Displacement Reactions • In displacement reactions, ions oxidize an element. • The ions, then, are reduced. Aqueous Reactions © 2009, Prentice-Hall, Inc. Displacement Reactions In this reaction, silver ions oxidize copper metal. Cu (s) + 2 Ag+ (aq) Cu2+ (aq) + 2 Ag (s) Aqueous Reactions © 2009, Prentice-Hall, Inc. Displacement Reactions The reverse reaction, however, does not occur. x Cu (s) + 2 Ag+ (aq) Cu2+ (aq) + 2 Ag (s) Aqueous Reactions © 2009, Prentice-Hall, Inc. • Metals often produce H2(g) when they react with acids. e.g. Mg(s) + 2HCl(aq) MgCl2(aq) + H2(g) • The metal is oxidized and the H+ is reduced. Aqueous Reactions © 2009, Prentice-Hall, Inc. • Metals may be oxidized in the presence of a salt: e.g. Fe(s)+ Ni(NO3)2(aq) Fe(NO3)2(aq)+ Ni(s) Net ionic equation: Fe(s) + Ni2+(aq) Fe2+(aq) + Ni(s) Fe has been oxidized to Fe2+ while Ni2+ has been reduced to Ni. Aqueous Reactions © 2009, Prentice-Hall, Inc. Sample Exercise 4.9 (p. 143) Write the balanced molecular and net ionic equations and half-reactions for the reaction of aluminum with hydrobromic acid. Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.9) Which of the following statements is true about the reaction between zinc and copper(II) sulfate? a) Zinc is oxidized, and copper ion is reduced. b) Zinc is reduced, and copper ion is oxidized. c) All reactants and products are soluble strong electrolytes. d) The oxidation state of copper in copper(II) sulfate is 0. e) More than one of the previous choices is Aqueous Reactions true. © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.9) Which of the following statements is true about the reaction between zinc and copper(II) sulfate? a) Zinc is oxidized, and copper ion is reduced. b) Zinc is reduced, and copper ion is oxidized. c) All reactants and products are soluble strong electrolytes. d) The oxidation state of copper in copper(II) sulfate is 0. e) More than one of the previous choices is Aqueous Reactions true. © 2009, Prentice-Hall, Inc. Practice Exercise 2 (4.9) a) Write the balanced molecular and net ionic equations for the reaction between magnesium and cobalt (II) sulfate. b) What is oxidized and what is reduced in the reaction? c) Write the half-reactions. Aqueous Reactions © 2009, Prentice-Hall, Inc. Activity Series Aqueous Reactions © 2009, Prentice-Hall, Inc. Sample Exercise 4.10 (p. 145) Will an aqueous solution of iron (II) chloride oxidize magnesium metal? If so, write the balanced molecular and net ionic equations for the reaction. Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.10) Which of these metals is the easiest to oxidize? a) gold b) lithium c) iron d) sodium e) aluminum Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.10) Which of these metals is the easiest to oxidize? a) gold b) lithium c) iron d) sodium e) aluminum Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 2 (4.10) Which of the following metals will be oxidized by Pb(NO3)2: Zn, Cu, Fe? Aqueous Reactions © 2009, Prentice-Hall, Inc. Molarity • Two solutions can contain the same compounds but be quite different because the proportions of those compounds are different. • Molarity is one way to measure the concentration of a solution. Molarity (M) = moles of solute L solution Aqueous Reactions © 2009, Prentice-Hall, Inc. Sample Exercise 4.11 (p. 146) Calculate the molarity of a solution made by dissolving 23.4 g of sodium sulfate (Na2SO4) in enough water to form 125 mL of solution. (1.32 M) Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.11) What is the molarity of a solution that is made by dissolving 3.68 g of sucrose (C12H22O11) in sufficient water to form 275.0 mL of solution? a) 13.4 M b) 7.43 x 10-2 M c) 3.91 x 10-2 M d) 7.43 x 10-5 M e) 3.91 x 10-5 M Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.11) What is the molarity of a solution that is made by dissolving 3.68 g of sucrose (C12H22O11) in sufficient water to form 275.0 mL of solution? a) 13.4 M b) 7.43 x 10-2 M c) 3.91 x 10-2 M d) 7.43 x 10-5 M e) 3.91 x 10-5 M Aqueous Reactions © 2009, Prentice-Hall, Inc. Mixing a Solution • To create a solution of a known molarity, one weighs out a known mass (and, therefore, number of moles) of the solute. • The solute is added to a volumetric flask, and solvent is added to the line on the neck of the flask. Aqueous Reactions © 2009, Prentice-Hall, Inc. Expressing the Concentration of an Electrolyte When an ionic compound dissolves, the relative concentrations of the ions in the solution depend on the chemical formula of the compound. e.g.: 1.0 M solution of NaCl: 1.0 M in Na+ ions and 1.0 M in Cl– ions. e.g.: 1.0 M solution of Na2SO4: 2.0 M in Na+ ions and 1.0 M in SO42– ions. Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 2 (4.11) Calculate the molarity of a solution made by dissolving 5.00 g of glucose (C6H12O6) in sufficient water to form exactly 100.0 mL of solution. (0.278 M) Aqueous Reactions © 2009, Prentice-Hall, Inc. Sample Exercise 4.12 (p. 148) What is the molar concentration of each ion present in a 0.025 M aqueous solution of calcium nitrate? (0.025 M Ca2+) 0.050 M NO3-) Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.12) What is the ratio of the concentration of potassium ions to the concentration of carbonate ions in a 0.015 M soluton of potassium carbonate? a) 1:0.015 b) 0.015:1 c) 1:1 d) 1:2 e) 2:1 Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.12) What is the ratio of the concentration of potassium ions to the concentration of carbonate ions in a 0.015 M soluton of potassium carbonate? a) 1:0.015 b) 0.015:1 c) 1:1 d) 1:2 e) 2:1 Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 2 (4.12) What is the molar concentration of K+ ions in a 0.015 M solution of potassium carbonate? (0.030 M) Aqueous Reactions © 2009, Prentice-Hall, Inc. Interconverting Molarity, Moles, and Volume • The definition of molarity contains three quantities: molarity, moles of solute, and liters of solution. • If we know any two of these, we can calculate the third. • Use dimensional analysis to help you. Aqueous Reactions © 2009, Prentice-Hall, Inc. Sample Exercise 4.13 (p. 146) How many grams of Na2SO4 are required to make 0.350 L of 0.500 M Na2SO4? (24.9 g) Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.13) What is the concentration of ammonia in a solution made by dissolving 3.75 g of ammonia in 120.0 L of water? a) 1.83 x 10-3 M b) 3.78 x 10-2 M c) 0.0313 M d) 1.83 M e) 7.05 M Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.13) What is the concentration of ammonia in a solution made by dissolving 3.75 g of ammonia in 120.0 L of water? a) 1.83 x 10-3 M b) 3.78 x 10-2 M c) 0.0313 M d) 1.83 M e) 7.05 M Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 2 (4.13) a) How many grams of Na2SO4 are there in 15 mL of 0.50 M Na2SO4? (1.1 g) b) How many milliliters of 0.50 M Na2SO4 solution are needed to provide 0.038 mol of this salt? (76 mL) Aqueous Reactions © 2009, Prentice-Hall, Inc. Dilution • One can also dilute a more concentrated solution by – Using a pipet to deliver a volume of the stock solution to a new volumetric flask, and – Adding solvent to the line on the neck of the new flask. Aqueous Reactions © 2009, Prentice-Hall, Inc. Dilution The molarity of the new solution can be determined from the equation Mc Vc = Md Vd, where Mc and Md are the molarity of the concentrated and dilute solutions, respectively, and Vc and Vd are the volumes of the two solutions. Aqueous Reactions © 2009, Prentice-Hall, Inc. Remember: Moles are central! Aqueous Reactions © 2009, Prentice-Hall, Inc. Example - Dilution How do you prepare 1.0 L of 2.0 M CuSO4 from a stock solution of 8.0 M CuSO4? First, figure out how much of the concentrated acid you need: Vconc = MdilVdil = (2.0 M)(1.0 L) = 0.25 L of 8.0 M CuSO4 Mconc (8.0 M) Aqueous Reactions © 2009, Prentice-Hall, Inc. Example - Dilution How do you prepare 1.0 L of 2.0 M CuSO4 from a stock solution of 8.0 M CuSO4? • Measure 250 mL of the concentrated (8.0 M) CuSO4, using either a graduated cylinder or a 250 mL volumetric flask. Pour into 1 L volumetric flask. • Add distilled water until total volume is 1000 mL or 1.0 L. Aqueous Reactions © 2009, Prentice-Hall, Inc. Sample Exercise 4.14 (p. 150) How many milliliters of 3.0 M H2SO4 are needed to make 450 mL of 0.010 M H2SO4? (1.5 mL) Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.14) What volume of a 1.00 M stock solution of glucose must be used to make 500.0 mL of a 1.75 x 10-2 M glucose solution in water? a) 1.75 mL b) 8.75 mL c) 48.6 mL d) 57.1 mL e) 28,570 mL Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.14) What volume of a 1.00 M stock solution of glucose must be used to make 500.0 mL of a 1.75 x 10-2 M glucose solution in water? a) 1.75 mL b) 8.75 mL c) 48.6 mL d) 57.1 mL e) 28,570 mL Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 2 (4.14) a) What volume of 2.50 M lead nitrate solution contains 0.0500 mol of Pb2+? (20.0 mL) b) How many milliliters of 5.0 M K2Cr2O7 solution must be diluted to prepare 250 mL of 0.10 M solution? (5.0 mL) c) If 10.0 mL of a 10.0 M stock solution of NaOH is diluted to 250 mL, what is the concentration of the resulting solution? (0.40 M) Aqueous Reactions © 2009, Prentice-Hall, Inc. Connections Where we have been: stoichiometry metathesis (precipitation and acid-base) reactions redox reactions molarity Aqueous Reactions © 2009, Prentice-Hall, Inc. Connections Now we are going to combine all these together: • apply stoichiometry to problems that contain molar concentrations • context = metathesis (precipitation and acid-base) reactions and redox reactions • practical application titrations • Reminder: MOLES ARE CENTRAL! Aqueous Reactions © 2009, Prentice-Hall, Inc. Using Molarities in Stoichiometric Calculations Recognize that there are two different types of units: Laboratory units (the macroscopic units that we measure in lab) and Chemical units (the microscopic units that relate to moles). Aqueous Reactions © 2009, Prentice-Hall, Inc. Using Molarities in Stoichiometric Calculations Aqueous Reactions © 2009, Prentice-Hall, Inc. Stoichiometry Reminder 1. 2. 3. 4. Write the equation Get to moles (of known) ASAP Switch to unknown via mole ratio Put answer in required unit Aqueous Reactions © 2009, Prentice-Hall, Inc. Sample Exercise 4.15 (p. 152) + more How many grams of calcium hydroxide are needed to neutralize 25.0 mL of 0.100 M HNO3? 1. Write the equation: 2 HNO3(aq) + Ca(OH)2(aq) Ca(NO3)2(aq) + 2 H2O(l) Aqueous Reactions © 2009, Prentice-Hall, Inc. Sample Exercise 4.15 (p. 152) + more 2. Get to moles (of known) ASAP (i.e. M mol, in this example) 3. Switch to moles of unknown, using mole ratio. 25.0 mL ( 1 L ) (0.100 mol HNO3)(1 mol Ca(OH)2) 103 mL 1L 2 mol HNO3 = 1.25 x 10-3 mol Ca(OH)2 Aqueous Reactions © 2009, Prentice-Hall, Inc. Sample Exercise 4.15 (p. 152) + more 4. a) How many grams of calcium hydroxide are needed to neutralize 25.0 mL of 0.100 M HNO3? (the original question) 1.25 x 10-3 mol Ca(OH)2 (74.10 g Ca(OH)2) 1 mol Ca(OH)2 = 9.26 x 10-2 g Ca(OH)2 Aqueous Reactions © 2009, Prentice-Hall, Inc. Sample Exercise 4.15 (p. 152) + more 4. b) How many mL of 0.50 M Ca(OH)2 are needed to completely neutralize 25.0 mL of 0.100 M HNO3? 1.25 x 10-3 mol Ca(OH)2( 1 L )(103 mL) 0.50 mol Ca(OH)2 1 L = 2.5 mL Aqueous Reactions © 2009, Prentice-Hall, Inc. Sample Exercise 4.15 (p. 152) + more 4. c) 15.0 mL of a Ca(OH)2 solution are needed to neutralize 25.0 mL of 0.100 M HNO3. What is the molarity of the solution? 1.25 x 10-3 mol Ca(OH)2 (103 mL) 15.0 mL 1L = 0.0833 M Ca(OH)2 Aqueous Reactions © 2009, Prentice-Hall, Inc. Sample Exercise 4.15 (p. 152) (see front board) How many grams of calcium hydroxide are needed to neutralize 25.0 mL of 0.100 M HNO3? (0.0926 g) Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.15) How many milligrams of sodium sulfide are needed to completely react with 25.00 mL of a 0.0100 M aqueous solution of cadmium nitrate, to form a precipitate of CdS(s)? a) 13.8 mg b) 19.5 mg c) 23.5 mg d) 32.1 mg e) 39.0 mg Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 2 (4.15) a) How many grams of NaOH are needed to neutralize 20.0 mL of 0.150 M H2SO4 solution? (0.240 g) b) How many liters of 0.500 M HCl(aq) are needed to react completely with 0.100 mol of Pb(NO3)2(aq), forming a precipitate of PbCl2(s)? (0.400 L) Aqueous Reactions © 2009, Prentice-Hall, Inc. Titration Titration is an analytical technique in which one can calculate the concentration of a solute in a solution. Aqueous Reactions © 2009, Prentice-Hall, Inc. Titration = A method of volumetric analysis in which a volume of one reagent is added to a known volume of another reagent slowly from a buret until an end point is reached. If one of the solutions has a known concentration, the concentration of the other can be calculated, via stoichiometry. Aqueous Reactions © 2009, Prentice-Hall, Inc. Titration Standard solution = reagent solution of known concentration Titrant = reagent solution of unknown concentration Equivalence point = the point at which stoichiometrically equivalent quantities are brought together End point = color change (v. close to equivalence point) Aqueous Reactions © 2009, Prentice-Hall, Inc. Change in appearance of a solution containing phenolphthalein as base indicator Aqueous Reactions © 2009, Prentice-Hall, Inc. Titration Aqueous Reactions © 2009, Prentice-Hall, Inc. Example Titration • Predict the number of mL of ~0.10 M NaOH needed to neutralize 10.0 mL of 0.25 M HCl. • We want to know the exact molarity of the NaOH solution. Aqueous Reactions © 2009, Prentice-Hall, Inc. Titration Practice Problems Remember: Moles are central! 1. In the titration of 35 mL of liquid drain cleaner containing NaOH, 50. mL of 0.40 M HCl must be added to reach the equivalence point. What is the molarity of the base in the cleaner? (0.57 M) Aqueous Reactions © 2009, Prentice-Hall, Inc. Titration Practice Problems Remember: Moles are central! 2. A 20.0 mL sample of an HCl solution is titrated with 27.4 mL of a standard solution of Ba(OH)2. The concentration of the standard is 0.0154 M. What is the molarity of the HCl? (0.0422 M) Aqueous Reactions © 2009, Prentice-Hall, Inc. Titration Practice Problems Remember: Moles are central! 3. How many mL of 0.25 M Ca(OH)2 must be added to titrate 46 mL of 0.40 M HClO4? (37 mL) Aqueous Reactions © 2009, Prentice-Hall, Inc. Titration Practice Problems Remember: Moles are central! 4. What is the molar hydrogen ion concentration in a 3.00 L solution of HNO3 in which 1.90 g of HNO3 is present? Assume the acid is a strong acid. What is its pH? (0.010 M, 2) molarity problem, not truly titration Aqueous Reactions © 2009, Prentice-Hall, Inc. Sample Exercise 4.16 (p. 153) One commercial method used to peel potatoes is to soak them in a solution of NaOH for a short time, remove them from the NaOH, and spry off the peel. The concentration of NaOH is normally in the range of 3 to 6 M. The NaOH is analyzed periodically. In one such analysis, 45.7 mL of 0.500 M H2SO4 is required to neutralize a 20.0 mL sample of NaOH solution. What is the concentration of the NaOH solution? (2.28 M) Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.16) What is the molarity of an HCl solution if 27.3 mL of it neutralizes 134.5 mL of 0.0165 M Ba(OH)2? a) 0.0444 M b) 0.0813 M c) 0.163 M d) 0.325 M e) 3.35 M Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.16) What is the molarity of an HCl solution if 27.3 mL of it neutralizes 134.5 mL of 0.0165 M Ba(OH)2? a) 0.0444 M b) 0.0813 M c) 0.163 M d) 0.325 M e) 3.35 M Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 2 (4.16) What is the molarity of an NaOH solution if 48.0 mL is needed to neutralize 35.0 mL of 0.144 M H2SO4? (0.210 M) Aqueous Reactions © 2009, Prentice-Hall, Inc. Sample Exercise 4.17 (p. 154) The quantity of Cl- in a water supply is determined by titrating the sample with Ag+: Ag+(aq) + Cl-(aq) AgCl(s) a) How many grams of chloride ion are in a sample of the water if 20.2 mL of 0.100 M Ag+ is needed to react with all the chloride in the sample? (7.17 x 10-2 g Cl-) b) If the sample has a mass of 10.0 g, what percent Cldoes it contain? (0.717% Cl-) Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.17) A mysterious white powder is found at a crime scene. A simple chemical analysis concludes that the powder is a mixture of sugar and morphine (C17H19NO3), a weak base similar to ammonia. The crime lab takes 10.00 mg of the mysterious white powder, dissolves it in 100.00 mL water, and titrates it to the equivalence point with 2.84 mL of a standard 0.0100 M HCl solution. What is the percentage of morphine in the white powder? a) 8.10% b) 17.3% c) 32.6% d) 49.7% Aqueous Reactions e) 81.0% © 2009, Prentice-Hall, Inc. Practice Exercise 1 (4.17) A mysterious white powder is found at a crime scene. A simple chemical analysis concludes that the powder is a mixture of sugar and morphine (C17H19NO3), a weak base similar to ammonia. The crime lab takes 10.00 mg of the mysterious white powder, dissolves it in 100.00 mL water, and titrates it to the equivalence point with 2.84 mL of a standard 0.0100 M HCl solution. What is the percentage of morphine in the white powder? a) 8.10% b) 17.3% c) 32.6% d) 49.7% Aqueous Reactions e) 81.0% © 2009, Prentice-Hall, Inc. Practice Exercise 2 (4.17) A sample of an iron ore is dissolved in acid, and the iron is converted to Fe2+. The sample is then titrated with 47.20 mL of 0.02240 M MnO4-. The oxidation-reduction reaction that occurs during titration is as follows: MnO4-(aq) + 5 Fe2+(aq) + 8 H+(aq) Mn2+(aq) + 5 Fe3+(aq) + 4 H2O(l) Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 2 (4.17) a) How many moles of MnO4- were added to the solution? (1.057 x 10-3 mol MnO4-) b) How many moles of Fe2+ were in the sample? (5.286 x 10-3 mol Fe2+) Aqueous Reactions © 2009, Prentice-Hall, Inc. Practice Exercise 2 (4.17) c) How many grams of iron were in the sample? (0.2952 g) d) If the sample had a mass of 0.8890 g, what is the percentage of iron in the sample? (33.21%) Aqueous Reactions © 2009, Prentice-Hall, Inc. Sample Integrative Exercise 4 (p. 155) A sample of 70.5 mg potassium phosphate is added to 15.0 mL of 0.050 M silver nitrate, resulting in the formation of a precipitate. Aqueous Reactions © 2009, Prentice-Hall, Inc. Sample Integrative Exercise 4 (p. 155) a) Write the molecular equation for the reaction. b) What is the limiting reactant in the reaction? c) Calculate the theoretical yield, in grams, of the precipitate that forms. (0.10 g Ag3PO4) Aqueous Reactions © 2009, Prentice-Hall, Inc.