* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Immune system wikipedia , lookup
Anti-nuclear antibody wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Complement system wikipedia , lookup
Innate immune system wikipedia , lookup
DNA vaccination wikipedia , lookup
Adaptive immune system wikipedia , lookup
Duffy antigen system wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Molecular mimicry wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
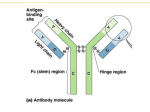
Figures replaced or skipped for Ch 2 • Figure 2.3 - Enzyme digestion of Ab’s • Flow cytometry - Fig 2.13 • Monoclonal Ab Fig 2.12 was replaced Chapter 2 Antibody Structure and the Generation of B-Cell Diversity • Molecular and structural basis of antibody diversity • How B cells develop and function in the body • How B cells are activated and participate in adaptive immunity Location and production of Immunoglobulins Humoral Immunity - immunity dominated by antibodies that can be transferred to another person 1. Antibodies are specific for individual epitopes 2. Membrane bound form is present on a B-cell 3. Ag binding to B cell stimulates it to secrete Ab Antibody structure Fab Fc Heavy (5 classes), Light (2 classes), Constant (C) and Variable (V) Disulfide bonds link heavy chains Fab- fragment antigen binding Fc- fragment crystallizable Immunoglobulin Isotypes or Classes pentamers N-linked carbohydrate Hinge region Disulfide bonds Monomer as BCR Multimeric forms k l - Light chain Ig domain Monomers or dimers Immunoglobulin (Ig) domain Figure 2-5 ~100 amino acid domain - very stable Two types Ig domains- variable and constant Antigen Binding site - VH and VL Heavy chain of IgG - four domains - VH, CH1, CH2, CH3 Figure 2-6 Anti-parallel -strands - contribute to the structural part Loop region - in the V domain contribute to variability Hypervariable Regions (CDR) of Antibodies HV - hypervariable regions CDR - complementarity determining regions Framework region - strand region that has reduced variability Amino Acid Sequence Variability in the V domain 110 amino acid lightchain V domain Framework regions can have variability but variability is much higher in the HV regions Physical Properties of Antigens-Antibody Binding Epitope - part of the antigen bound by Ab Antigen is usually carbohydrate or protein • Lock and Key concept • Variety of structures and sizes recognized by Ab’s • Affinity vs Avidity - terms describing binding strength of an antibody for its epitope Epitopes • Epitope (antigenic determinant) is the part of the antigen bound by Ab • Most antigens have multiple epitopes (multivalent) • Usually carbohydrate or peptide. Fig. 2.9 Mechanisms of Epitope Recognition • Linear and discontinuous epitopes • Multivalent Antigens • Polymeric Antibodies • Affinity vs Avidity - terms describing binding strength of an antibody for its epitope • Epitope binding mechanisms Figure 2-9 Figure 2-26 part 1 of 2 First Ab to be made during during an immune response to an antigen Monomers disulfide bonded together via the J-chain (joining chain) Figure 2-30 Monomers disulfide bonded to the J-chain Dimeric in mucosal lymphoid tissue - secreted into the gut to prevent pathogens binding to gut cells and can act as an antitoxin Monomeric made by B-cells in lymph nodes/spleen and is not J-chain dependent Antibody-antigen interaction • Non-covalent binding: – Electrostatic – Hydrogen bonds – Van der Waals force – Hydrophobic forces • Affinity: Strength of interaction between epitope and one antigen-binding site • Avidity: Strength of the sum of interactions between antibody and antigen Figure 2-8 Poliovirus VP1- blue, contains several epitopes (white) that can be recognized by human antibodies Antibodies bind a Range of Structures Pockets Molecule Grooves DNA Extended surfaces Lysozyme Examples of Problematic Ab binding to various structures Pocket: Penicillin - can bind to RBC surface proteins to create a foreign epitope. IgE binds to drug-RBC protein complex and initiates an inflammatory response Groove: DNA - Systemic Lupus Erythematosus (Lupus) - autoimmune disease in which antibodies are made against DNA and other molecules leading to inflammatory reactions in joints, skin and kidney Extended Surface: Lysozyme/Ovalbumin - allergies to hen egg components - more common in children under 5 - “desensitization” protocol can help Haptens Small molecules that are not immunogenic by themselves, but can bind immunoglobulins or TCRs. Haptens can induce an immune response when linked to a larger protein. Polyclonal vs Monoclonal Antibodies 1 4 A g 3 2 Isolate B-cells Spleen 1 2 4 3 Myeloma cells + Isolate serum Polyclonal antibodies 1 4 A g 3 2 Ab-1, Ab-2 Ab-3, Ab-4 Hybridoma Cells 1 2 3 4 1 2 3 4 Ab-1 Ab-2 Ab-3 Ab-4 Monoclonal antibodies Crossreactivity Occurs when an Antiserum is raised against antigen A but also reacts with antigen B Antigen A and B share epitopes Antigen A and B have similar (but not identical) epitopes Antibody Structure Summary • Produced by B-cells • Y shape, Four polypeptide chains, Ig domains • Constant and Variable regions • 5 classes - IgG, IgM, IgD, IgA, IgE • CDR, Hypervariable regions • Epitope recognition Examples of Commercial uses for Ab’s • • • • Pregnancy test Rh disease therapy Antitoxin serum - Antivenin, rabies, etc Anti-cancer monoclonals - will be covered later in the course Pregnancy test Input window Reaction zone Pregnancy window Control window Urine Mouse Monoclonal anti-HCG Ab-enzyme conjugate Immobile Polyclonal anti-HCG Ab + dye substrate Immobile Goat Antimouse Ab + dye substrate http://mcb.berkeley.edu/courses/mcb150/lecture5/Preg%20Test%20movie.swf Rh disease Rh disease (Erythroblastosis Fetalis) - Hemolytic disease of a newborn - occurs in Rhwoman carrying Rh+ fetus RhoGAM - human plasma with anti-Rh+ (D antigen) antibodies. Works by binding any fetal RBC’s before the mother is able to produce an immune response and form anti-D IgG Antitoxin serum • Serum can be used in the prophylaxis and/or treatment of rabies, botulism, diphtheria, gas gangrene, snake and spider bites • Antivenin Crotalidae Polyvalent (ACP) - horse serum based antivenom - gold standard for snake bites but can cause “serum sickness” • New types of antitoxins – Fragmented antivenin (CroFab) where only the Fab fragment (sheepbased) is used – Humanized antibodies derived from mouse sources Generation of Ig diversity in B cells Unique organization - Only B cells can express Ig protein - Gene segments k l - Light chain , , , , - Heavy Chain present on three chromosomes The Gene Rearrangement Concept • Germline configuration • Gene segments need to be reassembled for expression • Sequentially arrayed • Occurs in the B-cells precursors in the bone marrow (soma) • A source of diversity BEFORE exposure to antigen V-variable, J-joining, D-diversity gene segments; L-leader sequences Light Chain Variable - V, J Constant - C Heavy Chain Variable - V, D, J Constant - C Gene rearrangements during B-cell development V-variable, J-joining, D-diversity gene segments; L-leader sequences Figure 2-14 l - 30 V & 4 pairs J & C (light chain) chs22 k – 40 V & 5 J & 1 C (50% have 2x V) (light chain) chs2 H – 65 V & 27 D & 6 J chs14 V-region of light chain consists of one V and one J segment C-region is encoded by one C segment Variability comes from the V segment CDR1 and CDR2 CDR3 D-diversity CDR1 and CDR2 CDR3 Two recombination events needed to combine 3 segments to make the Variable region Random Recombination of Gene segments is one factor contributing to diversity (200* + 120*) X 10,530 = 3,369,600 Ig molecules * Only one light chain loci gives rise to one functional polypeptide! Mechanism of Recombination • Recombination signal sequences (RSSs) direct recombination - V and J (L chain) - V D J (H chain) • RSS types consist of: - nonamer (9 base pairs) - heptamer (7 base pairs) - Spacer - Two types: [7-12-9], [7-23-9] • RSS features: - recognition sites for recombination enzymes - recombination occurs in the correct order (12/23 rule) Mechanism of Somatic Recombination • V(D)J recombinase: all the protein components that mediate the recombination steps • RAG complex: Recombination Activating Genes (RAG-1 and RAG2) encode RAG proteins only made in lymphocytes • Recombination only occurs through two different RSS bound by two RAG complexes (12/23 rule) • DNA cleavage occurs to form a single stranded hairpin and a break at the heptamer sequences • Enzymes that cut and repair the break introduce Junctional Diversity Recombination + Junctional Diversity http://www.blink.biz/immunoanimations/# Figure 2-19 Junctional Diversity Figure 2-18 part 2 of 3 • Nucleotides introduced at recombination break in the coding joint corresponding to CDR3 of light and heavy chains - V and J of the light chain - (D and J) or (V and DJ) of the heavy chain • P nucleotides generate short palindromic sequences • N nucleotides are added randomly - these are not encoded • Junctional Diversity contributes 3 x107 to overall diversity! Generation of BCR (IgD and IgM) • Rearrangement of VDJ of the heavy chain brings the gene’s promoter closer to C and C • Both IgD and IgM are expressed simultaneously on the the surface of the B cell as BCR - ONLY isotypes to do this • Alternative splicing of the primary transcript RNA generates IgD and IgM • Naïve B cells are early stage B cells that have yet to see antigen and produce IgD and IgM Alternative Splicing of Primary Transcript to generate IgM or IgD Figure 2-21 Summary Biosynthesis of IgM in B cells Mature B cell Figure 2-23 • Long cytoplasmic tails interact with intracellular signaling proteins • Disulfide-linked • Ig and Ig - invariant - Transmembrane proteins - Dual-function 1) help the assembled Ig reach the cell surface from the ER 2) signal the B cell to divide and differentiate Principle of Single Antigen Specificity • Each B cell contains two copies of the Ig locus (Maternal and Paternal copies) • Only one is allowed to successfully rearrange - Allelic Exclusion l * Chromosome 22 k H 2 14 * • All Igs on the surface of a single B cell have identical specificity and differ only in their constant region • Result: B cell monospecificity means that a response to a pathogen can be very specific DNA hybridization of Ig genes can diagnose B-cell leukemias Peripheral blood from healthy patient is made up of mostly neutrophils Peripheral blood from a leukemia patient has an abnormally high proportion of B-cells. Cancer cells are derived from one clonal line of B-cell which has V and C chains rearranged next to each other Generation of B cell diversity in Ig’s before Antigen Encounter 1. Random combination of V and J (L chain) and V, D, J (H chain) regions 2. Junctional diversity caused by the addition of P and N nucleotides 3. Combinatorial association of Light and Heavy chains (each functional light chain is found associated with a different functional heavy chain and vice versa) Concept of Combinatorial Association Developmental stages of B cells 1. Development before antigen Immature B cell 2. Development after antigen Mature naive B cell (expressing BCR IgM and IgD) plasma cell (expressing BCR and secretes Antibodies) Processes occurring after B cells encounter antigen • Processing of BCR versus Antibody 1. Plasma cells switch to secreted Ab 2. Difference occurs in the c-terminus of the heavy chain 3. Primary transcript RNA is alternatively processed to yield transmembrane or secreted Ig’s • Somatic Hypermutation 1. Point mutations introduced to V regions 2. 106 times higher mutation rate 3. Usually targets the CDR • Affinity maturation - mutant Ig molecules with higher affinity are more likely to bind antigen and their B cells are preferentially selected • Isotype switching RNA processing to generate BCR or Antibody MC - membrane coding SC - secretion coding Somatic Hypermutation (random introduction of point mutations) Mutations occur throughout the V domain - especially CDR Occurs on both gene copies - but only one expresses protein AID - Activation induced Cytidine Deaminase UNG - Uracil-DNA glycosylase Process of Affinity Maturation Hypermutation leads to different B-cells IgM Hypermutation Mutant BCRs have various affinities Higher affinity BCR’s are preferentially selected to mature IgG IgM Isotype switching 1. IgM is the first Ab that is secreted in the IR 2. IgM is pentameric and each H chain can bind complement proteins 3. Isotypes with better effector functions are produced by activated B cells 4. Rearrangement of DNA using SWITCH regions - all C genes preceded by switch sequence (except - start from the gene and any other C gene (plus sequential) 5. Regulated by cytokines secreted by T cells Switch regions flank each C gene (except delta) Mu to any other isotype Sequential switching AID is important AID deficiency ---> Hyper IgM syndrome (antibodies not made after IgM and IgD ) Stages at which Isotype Switching occurs antigen-independent stem cell pre-B cell immature B cell (IgM +) mature B cell (IgM +, IgD +) antigen-dependent IgM isotype switching IgG IgE IgA Does Isotype Switching occur in one B cell? 1. Activated B cell resides in the Germinal Center -some individuals will mature directly into plasma cells 2. Some B cells in the germinal center divide and undergo hypermutation and/or isotype switching 3. After this stage they cannot divide and the higher affinity ones are selected 4. These cells can mature to plasma cells 5. End result: The B cell makes a different antibody isotype but with the same specificity Immunoglobulin classes Figure 2-31 part 2 of 2 1. C regions determine the class of antibody and their effector function 2. Divided into Subclasses based on relative abundance in serum 3. Each class has multiple functions 1. IgM and IgG can bind complement 2. IgG crosses placenta 3. Receptors for constant regions (Fc Receptors) - IgG (FcG receptors): mac, neutrophils, eosinophils, NK cells, others - IgE (FcE receptors): mast cells, basophils, others Initial Immune Response mediated by IgM IgM (plasma cells in lymph nodes, spleen, and bone marrow and circulate in blood/lymph) Low affinity binding to antigen via multiple binding sites Two binding sites sufficient for strong binding Hypermutation and affinity maturation Exposure of constant region Activate complement Phagocytose Isotype switching to IgG Kill directly IgG (lymph nodes, spleen, and bone marrow) Circulates in blood and lymph (most abundant Ab in internal fluids) Extravasation, Higher affinity binding to antigen Recruit phagocytes Multiple effector functions Neutralize antigens Activate complement Monomeric IgA Plasma cells in lymph nodes, spleen, bone marrow Dimeric IgA lymphoid tissue associated with mucosal surfaces Secreted into Blood Secreted into Gut lumen & body secretions IgA most made of any Ab Effector functions 1. Mainly neutralization 2. Minor opsonization and activation of complement IgE Plasma cells in lymph nodes or germinal centers Bind strongly to Mast cells via Fc receptor Inflammation - Expulsion of large pathogens - Allergies Cross-linking of receptor bound Ab releases histamine and other activators IgD • Very low concentration in serum • Primarily found with IgM on naïve mature B cells • Function is not clear Figure 2-32 Summary: Generation of B-cell diversity • Diversity before Antigen exposure (Antigen Independent) - Random Recombination - Junctional Diversity - Combinatorial association • Diversity after Antigen exposure (Antigen Dependent) - Switch to secreted Ab - Somatic Hypermutation - Affinity Maturation - Isotype Switching • Immunoglobulin Classes - Properties - Effector functions