* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download FoundationsofChemistryppt
Chemical equilibrium wikipedia , lookup
Thermodynamics wikipedia , lookup
Destruction of Syria's chemical weapons wikipedia , lookup
Chemical bond wikipedia , lookup
Chemical warfare wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Chemical element wikipedia , lookup
Atomic nucleus wikipedia , lookup
California Green Chemistry Initiative wikipedia , lookup
Photopolymer wikipedia , lookup
Ceramic engineering wikipedia , lookup
Chemical reaction wikipedia , lookup
Thermomechanical analysis wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Stoichiometry wikipedia , lookup
Drug discovery wikipedia , lookup
Nanochemistry wikipedia , lookup
Transition state theory wikipedia , lookup
Al-Shifa pharmaceutical factory wikipedia , lookup
Chemical industry wikipedia , lookup
Chemical weapon proliferation wikipedia , lookup
History of molecular theory wikipedia , lookup
Chemical weapon wikipedia , lookup
Chemical plant wikipedia , lookup
Condensed matter physics wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Chemical Corps wikipedia , lookup
Molecular dynamics wikipedia , lookup
Chemical potential wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
Safety data sheet wikipedia , lookup
History of chemistry wikipedia , lookup
VX (nerve agent) wikipedia , lookup
Atomic theory wikipedia , lookup
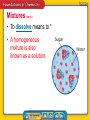
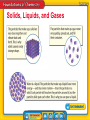
Chapter Introduction Lesson 1 Classifying Matter Lesson 2 Physical Properties Lesson 3 Physical Changes Lesson 4 Chemical Properties and Changes Chapter Wrap-Up What is matter, and how does it change? What do you think? Before you begin, decide if you agree or disagree with each of these statements. As you view this presentation, see if you change your mind about any of the statements. Do you agree or disagree? 1. The atoms in all objects are the same. 2. You cannot always tell by an object’s appearance whether it is made of more than one type of atom. 3. The weight of a material never changes, regardless of where it is. 4. Boiling is one method used to separate parts of a mixture. Do you agree or disagree? 5. Heating a material decreases the energy of its particles. 6. When you stir sugar into water, the sugar and water evenly mix. 7. When wood burns, new materials form. 8. Temperature can affect the rate at which chemical changes occur. Classifying Matter • What is a substance? • How do atoms of different elements differ? • How do mixtures differ from substances? • How can you classify matter? Classifying Matter • matter • mixture • atom • heterogeneous mixture • substance • element • homogeneous mixture • compound • dissolve Understanding Matter Matter * matter from Latin materia, meaning “material, stuff” Understanding Matter (cont.) • Everything you can see is matter, but some things you cannot not see, like air, are also matter. • An atom is a * Atoms • At the center of an atom is the nucleus. • Protons, which have a positive charge, and neutrons, which have no charge, make up the nucleus. Atoms (cont.) • Negatively charged particles, or electrons, move quickly throughout an area around the nucleus called the electron cloud. • Not all atoms have the same number of protons, neutrons, and electrons. Substances • A substance is *. • This means that a given substance is always made up of one or more atoms in the same combinations. • Two type of substances are elements and compounds. Substances (cont.) • An element is a *. • Because there are about 115 known elements, there are about 115 different types of atoms. • Each type of atom contains a different number of protons in its nucleus. The number of protons in an atom is the atomic number of the element. Each element on the periodic table consists of just one type of atom. Substances (cont.) • A compound is a * • The combination of symbols and numbers that represents a compound is called a chemical formula. If a substance contains only one type of atom, it is an element. If it contains more than one type of atom, it is a compound. Substances (cont.) • Chemical formulas show the different atoms that make up a compound, using their element symbols. • Chemical formulas also help explain how the atoms combine. • A compound often has different properties from the individual elements that compose it. Mixtures • A mixture is * • Mixtures are combinations of two or more substances that are physically blended together. • The amounts of the substances can vary in different parts of a mixture and from mixture to mixture. Mixtures (cont.) • A heterogeneous mixture is * • Because the substances in a heterogeneous mixture are not evenly mixed, two sample of the same mixture can have different amounts of the substances. Mixtures (cont.) • A homogeneous mixture is * • In a homogeneous mixture, the particles of individual substances are so small and well-mixed that they are not visible, even with most high-powered microscopes. Mixtures (cont.) • To dissolve means to * • A homogeneous mixture is also known as a solution. Compounds v. Solutions • The composition in a compound does not vary. Therefore, a chemical formula can be used to describe the compound. • Because composition in a mixture can vary, a chemical formula cannot be used to describe mixtures. • A substance has the same composition throughout. A substance is either an element or a compound. • An atom is the smallest part of an element that has its properties. Atoms contain protons, neutrons, and electrons. • The substances in a mixture are not chemically combined. Mixtures can be either heterogeneous or homogeneous. Physical Properties • What are some physical properties of matter? • How are physical properties used to separate mixtures? Physical Properties • physical property • mass • density • solubility Physical Properties • A physical property is * • Solids, liquids, and gases are called states of matter. • Every solid, liquid, and gas around you is made up of moving particles that attract one another. Solids, Liquids, and Gases Physical Properties (cont.) • Mass is * • Volume is the amount of space something takes up. • Mass and volume are size-dependent properties of matter because their values depend on the size of a sample. Physical Properties (cont.) • The temperature at which a substance changes from a solid to a liquid is its melting point. • The temperature at which a substance changes from a liquid to a gas is its boiling point. • Melting point and boiling point are sizeindependent properties—properties that are the same for both small samples and large samples. The boiling point of water is 100°C at sea level. The boiling point does not change for different volumes of water. Physical Properties (cont.) Density is *. density from Latin densus, means “compact”; and Greek dasys, means “thick” Physical Properties (cont.) • Electrical conductivity is the ability of matter to conduct, or carry along, an electric current. • Thermal conductivity is the ability of a material to conduct thermal energy. • Solubility is the * Physical Properties (cont.) Physical Properties (cont.) Physical Properties (cont.) The substances that make up mixtures are not held together by chemical bonds. bond Science Use a force between atoms or groups of atoms Common Use a monetary certificate issued by a government or a business that earns interest The parts of a mixture often can be separated by physical properties. Physical Properties (cont.) Physical properties cannot be used to separate a compound into the elements it contains. How are physical properties used to separate mixtures? • A physical property is a characteristic of matter that can be observed or measured without changing the identity of the matter. • Examples of physical properties include mass, density, volume, melting point, boiling point, state of matter, and solubility. • Many physical properties can be used to separate the components of a mixture. Physical Changes • How can a change in energy affect the state of matter? • What happens when something dissolves? • What is meant by conservation of mass? Physical Changes • physical change Physical Changes • A physical change is a *. • During a physical change, the matter does not become something different even though physical properties change. Physical Changes (cont.) physical from Greek physika, means “natural things” change from Latin cambire, means “to exchange” Physical Changes (cont.) • When thermal energy is added to a solid, the particles in the solid move faster and faster, and the temperature increases. • When the particles are moving too fast for attractive forces to hold them tightly together, the solid reaches its melting point. Physical Changes (cont.) • When the particles in a liquid are moving so fast that attractive forces cannot hold them close together, the liquid reaches its boiling point. • Sublimation occurs when a solid changes directly to a gas without first becoming a liquid. As thermal energy is added to a material, temperature increases when the state of the material is not changing. Temperature stays the same during a change of state. Physical Changes (cont.) • When thermal energy is removed from a gas, particles in the gas move more slowly and the temperature decreases. • Condensation occurs when the particles are moving slowly enough for attractive forces to pull the particles close together. • After the gas has completely changed to a liquid, removing more thermal energy from the liquid causes particles move even more slowly. Physical Changes (cont.) • Freezing occurs when the particles are moving so slowly that attractive forces between the particles hold them tightly together. • Deposition is the change from a gas directly to a solid. Physical Changes (cont.) Dissolving, or evenly mixing, is a physical change because the identities of the substances involved do not change. What happens when something dissolves? Conservation of Mass Conservation of mass refers to the fact that the total mass before and after a physical change is the same. • During a physical change, matter can change form, shape, size, or state, but the identity of the matter does not change. • Matter either changes temperature or changes state when enough thermal energy is added or removed. • Mass is conserved during physical changes, which means that mass is the same before and after the changes occur. Chemical Properties and Changes • What is a chemical property? • What are some signs of chemical change? • Why are chemical equations useful? • What are some factors that affect the rate of chemical reactions? Chemical Properties and Changes • chemical property • chemical change • concentration Chemical Properties • A chemical property is * • Chemical properties include ability to burn, acidity, and ability to rust. Comparing Properties • All matter can be described using both physical and chemical properties. • The ability of a substance to burn or rot is a chemical property. What are some chemical properties of matter? Chemical Changes • A chemical change is a change in matter in which * • The substances that undergo a chemical change no longer have the same properties because they no longer have the same identity. Signs of Chemical Change • Signs of chemical changes include the formation of bubbles or a change in odor, color, or energy. • These signs do not always mean a chemical change occurred. • The only proof of chemical change is the formation of a new substance. Explaining Chemical Reactions • Chemical changes often are called chemical reactions. • A useful way to understand what happens during a chemical reaction is to write a chemical equation. • A chemical equation shows the chemical formula of each substance in the reaction. Explaining Chemical Reactions (cont.) Chemical formulas and other symbols are parts of a chemical equation. Explaining Chemical Reactions (cont.) • The formulas to the left of the arrow represent the reactants—the substances present before the reaction takes place. • The formulas to the right of the arrow represent the products—the new substances present after the reaction. • The arrow indicates that a reaction has taken place. Explaining Chemical Reactions (cont.) • In a chemical equation, the number of atoms of each element before a reaction must equal the number of atoms of each element after the reaction. • This is called a balanced chemical equation, and it illustrates the conservation of mass. • When balancing an equation, you cannot change the chemical formula of any reactants or products. The Rate of Chemical Reactions • Different factors can make particles move faster and collide harder and more frequently, increasing the rate of chemical reaction. • A higher temperature usually increases the rate of reaction because when the temperature is higher, the particles move faster. The Rate of Chemical Reactions (cont.) • Concentration is * • Surface area also affects reaction rate if at least one reactant is a solid. Chemistry • To understand chemistry, you need to understand matter and how the arrangement of atoms results in different types of matter. • You also need to be able to distinguish physical properties from chemical properties and describe ways these properties can change. • A chemical property is observed only as a material undergoes chemical change and changes identity. • Signs of possible chemical change include bubbles, energy change, and change in odor or color. • Chemical equations show the reactants and products of a chemical reaction and that mass is conserved. Matter is anything that has mass and takes up space. Its physical properties and its chemical properties can change. Lesson 1: Classifying Matter • A substance is a type of matter that always is made of atoms in the same combination. • Atoms of different elements have different numbers of protons. • The composition of a substance cannot vary. The composition of a mixture can vary. • Matter can be classified as either a substance or a mixture. Lesson 2: Physical Properties • Physical properties of matter include size, shape, texture, and state. • Physical properties such as density, melting point, boiling point, and size can be used to separate mixtures. Lesson 3: Physical Changes • A change in energy can change the state of matter. • When something dissolves, it mixes evenly in a substance. • The masses before and after a change in matter are equal. Lesson 4: Chemical Properties and Changes • Chemical properties include ability to burn, acidity and ability to rust. • Some signs that might indicate chemical changes are the formation of bubbles and a change in odor, color, or energy. • Chemical equations are useful because they show what happens during a chemical reaction. • Some factors that affect the rate of chemical reactions are temperature, concentration, and surface area.