Chemical Dynamics at Surfaces
... Hydrogen production using electrolysis of water powered by renewable energy is not yet costcompetitive with hydrogen from fossil fuels, such as natural gas, and so has been responsible for only 4% of current hydrogen production. ...
... Hydrogen production using electrolysis of water powered by renewable energy is not yet costcompetitive with hydrogen from fossil fuels, such as natural gas, and so has been responsible for only 4% of current hydrogen production. ...
TEKS 8 - UNT College of Education
... A chemical reaction, also called a chemical change, is material changing from a beginning mass to a resulting substance. The process involves one or more reactants yielding one or more products different from the reactants. The characteristic of a chemical reaction is that new material or materials ...
... A chemical reaction, also called a chemical change, is material changing from a beginning mass to a resulting substance. The process involves one or more reactants yielding one or more products different from the reactants. The characteristic of a chemical reaction is that new material or materials ...
Chapter 19 Chemical Thermodynamics
... First Law of Thermodynamics • You will recall from Chapter 5 that energy cannot be created nor destroyed. • Therefore, the total energy of the universe is a constant. • Energy can, however, be converted from one form to another or transferred from a system to the surroundings or vice versa. Chemica ...
... First Law of Thermodynamics • You will recall from Chapter 5 that energy cannot be created nor destroyed. • Therefore, the total energy of the universe is a constant. • Energy can, however, be converted from one form to another or transferred from a system to the surroundings or vice versa. Chemica ...
Diversity-oriented synthesis - David Spring
... The General Requirements of a DOS The members of a DOS library should be diverse in both the appendages they display and also in the three-dimensional orientations and locations of these appendages.12 Thus, in designing a DOS, strategies to incorporate the four key types of diversity must be include ...
... The General Requirements of a DOS The members of a DOS library should be diverse in both the appendages they display and also in the three-dimensional orientations and locations of these appendages.12 Thus, in designing a DOS, strategies to incorporate the four key types of diversity must be include ...
Chapter 19 Chemical Thermodynamics
... by both enthalpy and entropy. • Gibb’s Free Energy is a thermodynamic function that combines enthalpy and entropy. • For a reaction occurring at constant pressure and temperature, the sign of Gibb’s Free Energy relates to the spontaneity of the ...
... by both enthalpy and entropy. • Gibb’s Free Energy is a thermodynamic function that combines enthalpy and entropy. • For a reaction occurring at constant pressure and temperature, the sign of Gibb’s Free Energy relates to the spontaneity of the ...
Theories in the Evolution of Chemical Equilibrium: Impli
... The earliest affinity table was that published by E.F. Geoffroy in 1718 (4). This table consists of sixteen columns. At the head of each column there is the traditional symbol of a substance (or a group of substances). Below it, the symbols for the substances with which it reacts are arranged in ord ...
... The earliest affinity table was that published by E.F. Geoffroy in 1718 (4). This table consists of sixteen columns. At the head of each column there is the traditional symbol of a substance (or a group of substances). Below it, the symbols for the substances with which it reacts are arranged in ord ...
ppt
... First Law of Thermodynamics • You will recall from Chapter 5 that energy cannot be created nor destroyed. • Therefore, the total energy of the universe is a constant. • Energy can, however, be converted from one form to another or transferred from a system to the surroundings or vice versa. Chemica ...
... First Law of Thermodynamics • You will recall from Chapter 5 that energy cannot be created nor destroyed. • Therefore, the total energy of the universe is a constant. • Energy can, however, be converted from one form to another or transferred from a system to the surroundings or vice versa. Chemica ...
Chapter 19 Chemical Thermodynamics
... • Energy can, however, be converted from one form to another or transferred from a system to the surroundings or vice versa. Chemical Thermodynamics ...
... • Energy can, however, be converted from one form to another or transferred from a system to the surroundings or vice versa. Chemical Thermodynamics ...
Chemical Reaction
... 2. When a chemical reaction takes place, the products can be turned back into the reactants… a) very easily. b) with difficulty. c) with a magic wand. © OUP: To be used solely in purchaser’s school or college ...
... 2. When a chemical reaction takes place, the products can be turned back into the reactants… a) very easily. b) with difficulty. c) with a magic wand. © OUP: To be used solely in purchaser’s school or college ...
LESSON 23: Exploding Bags
... for performing the lesson or similar lessons. They also introduce ways to expand on the content topics presented and think beyond those topics. Use the following examples, or have a discussion to generate other ideas as a class. • Ask the students what they would expect to happen if the amount of ba ...
... for performing the lesson or similar lessons. They also introduce ways to expand on the content topics presented and think beyond those topics. Use the following examples, or have a discussion to generate other ideas as a class. • Ask the students what they would expect to happen if the amount of ba ...
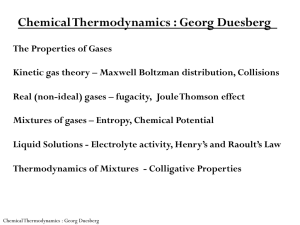
Chemical Thermodynamics : Georg Duesberg
... Physical properties of gases can be described by motion of individual gas atoms/molecules Assumptions: 1) each macroscopic and microscopic particle in motion holds an kinetic energy according to Newton’s law 2) They undergo elastic collisions 3) They are large in number and are randomly distributed ...
... Physical properties of gases can be described by motion of individual gas atoms/molecules Assumptions: 1) each macroscopic and microscopic particle in motion holds an kinetic energy according to Newton’s law 2) They undergo elastic collisions 3) They are large in number and are randomly distributed ...
Chapter 8
... • List three observations that suggest that a chemical reaction has taken place. • List three requirements for a correctly written chemical equation. • Write a word equation and a formula equation for a given chemical reaction. • Balance a formula equation by inspection. ...
... • List three observations that suggest that a chemical reaction has taken place. • List three requirements for a correctly written chemical equation. • Write a word equation and a formula equation for a given chemical reaction. • Balance a formula equation by inspection. ...
L2004-01A
... mimic the movement of real fish and are equipped with chemical sensors to sniff out potentially hazardous pollutants, such as leaks from vessels or underwater pipelines. They will transmit the information back to shore using Wi-Fi technology. Unlike earlier robotic fish, which needed remote controls ...
... mimic the movement of real fish and are equipped with chemical sensors to sniff out potentially hazardous pollutants, such as leaks from vessels or underwater pipelines. They will transmit the information back to shore using Wi-Fi technology. Unlike earlier robotic fish, which needed remote controls ...
Comparison of 2008 to 2000 SCH3U_ud
... compounds can be released into the environment, including cadmium, arsenic, sulphur dioxide, and mercury, all of which can endanger the health and safety of local populations. Sample questions: What are some chemical reactions used in the manufacture of paper? How might the reactants or products of ...
... compounds can be released into the environment, including cadmium, arsenic, sulphur dioxide, and mercury, all of which can endanger the health and safety of local populations. Sample questions: What are some chemical reactions used in the manufacture of paper? How might the reactants or products of ...
Role of Chemistry in Everyday Life
... environment while adding carbon dioxide and water. Plants use these to carry out photosynthesis, while releasing oxygen and water again out of their leaves. The ph of various things in the home have to do with chemistry from the acidic orange juice to the alkaline bleach. When you eat foods, hydroly ...
... environment while adding carbon dioxide and water. Plants use these to carry out photosynthesis, while releasing oxygen and water again out of their leaves. The ph of various things in the home have to do with chemistry from the acidic orange juice to the alkaline bleach. When you eat foods, hydroly ...
FoundationsofChemistryppt
... • Because there are about 115 known elements, there are about 115 different types of atoms. • Each type of atom contains a different number of protons in its nucleus. The number of protons in an atom is the atomic number of the element. ...
... • Because there are about 115 known elements, there are about 115 different types of atoms. • Each type of atom contains a different number of protons in its nucleus. The number of protons in an atom is the atomic number of the element. ...
Chemical Technology - Engineers Institute of India
... 1. Chemical and allied Industry have first rank among all manufacturing industry both in capital assets and importance to the country economy. 2. Chemical Industry plays important role in every part of life. For example foods, drugs, petroleum, and fertilizer industry 3. Chemical Industry is differe ...
... 1. Chemical and allied Industry have first rank among all manufacturing industry both in capital assets and importance to the country economy. 2. Chemical Industry plays important role in every part of life. For example foods, drugs, petroleum, and fertilizer industry 3. Chemical Industry is differe ...
chemical reaction
... are never lost or gained in a chemical reaction, they are just rearranged. Every atom in the reactants becomes part of the products. • When writing a chemical equation, make sure the number of atoms of each element in the reactants equals the number of atoms of those same elements in the products. T ...
... are never lost or gained in a chemical reaction, they are just rearranged. Every atom in the reactants becomes part of the products. • When writing a chemical equation, make sure the number of atoms of each element in the reactants equals the number of atoms of those same elements in the products. T ...
Chapter 1 Matter and Change
... cooking: a) the food darkens, or b) bubbles form in boiling water? Which of the following is used for chemical symbols today: a) letters, or b) drawings? When an iron nail is ground into powder, its mass ____. –Stays the same ...
... cooking: a) the food darkens, or b) bubbles form in boiling water? Which of the following is used for chemical symbols today: a) letters, or b) drawings? When an iron nail is ground into powder, its mass ____. –Stays the same ...
the powerpoint
... • When a substance changes color, the chemical composition of the substance may have changed (for example, iron turns to a reddish-brown when it rusts, clothes change color when bleach is added, apples turn brown when they react with oxygen in the air, or marshmallows turn black when burned). • It i ...
... • When a substance changes color, the chemical composition of the substance may have changed (for example, iron turns to a reddish-brown when it rusts, clothes change color when bleach is added, apples turn brown when they react with oxygen in the air, or marshmallows turn black when burned). • It i ...
___Mg + ___O ___MgO • Mole : Mole ratio
... reacts with nitrogen gas to form 20.4 grams of ammonia (nitrogen trihydride)? 2) What is the percent yield when 2.37 grams of silver nitrate reacts with sodium hydroxide to produce water, sodium nitrate and 1.55 grams of silver oxide? ...
... reacts with nitrogen gas to form 20.4 grams of ammonia (nitrogen trihydride)? 2) What is the percent yield when 2.37 grams of silver nitrate reacts with sodium hydroxide to produce water, sodium nitrate and 1.55 grams of silver oxide? ...
Lecture 14
... A compound is 24.27% C, 4.07% H, and 71.65% Cl. The molar mass is known to be 99.0 g. What are the empirical and molecular formulas? ...
... A compound is 24.27% C, 4.07% H, and 71.65% Cl. The molar mass is known to be 99.0 g. What are the empirical and molecular formulas? ...
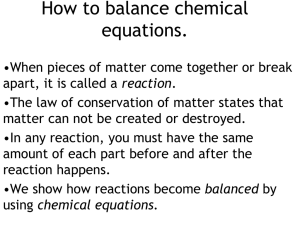
How to balance chemical equations.
... •When pieces of matter come together or break apart, it is called a reaction. •The law of conservation of matter states that matter can not be created or destroyed. •In any reaction, you must have the same amount of each part before and after the reaction happens. •We show how reactions become balan ...
... •When pieces of matter come together or break apart, it is called a reaction. •The law of conservation of matter states that matter can not be created or destroyed. •In any reaction, you must have the same amount of each part before and after the reaction happens. •We show how reactions become balan ...
Chemical Properties - Michigan State University
... what is occurring in the lab. How has the sugar changed? (asked after the physical AND chemical change) Is the sugar still present? How are the physical and chemical changes different? How would you classify a physical change? What about a chemical change? I want to discuss and ask a question also a ...
... what is occurring in the lab. How has the sugar changed? (asked after the physical AND chemical change) Is the sugar still present? How are the physical and chemical changes different? How would you classify a physical change? What about a chemical change? I want to discuss and ask a question also a ...